HOME >> BUSINESS
Top vaccine firms fail to get permit
By Chen Tian Source:Global Times Published: 2014-1-1 23:43:02
A nurse vaccinates a baby at a hospital in Taiyuan, North China's Shanxi Province. Photo: IC
China's top three producers of hepatitis B vaccine have failed to obtain the government's authorization to manufacture new products this year, which may cause a shortage in the hepatitis B vaccine market, analysts said Wednesday.
The China Food and Drug Administration (CFDA), the country's top food and drug watchdog, did not include Dalian Hissen BioPharm Co, Shenzhen BioKangtai and Beijing Tiantan Biological Products Corporation in its most updated list released Monday of companies that have received the Good Manufacturing Practices (GMP) certificate for pharmaceutical products.
The country's current GMP rules, which were adopted in early 2011, include stricter requirements for pharmaceutical companies in terms of their manufacturing process and the quality of their production facilities, according to a notice the CFDA released Tuesday.
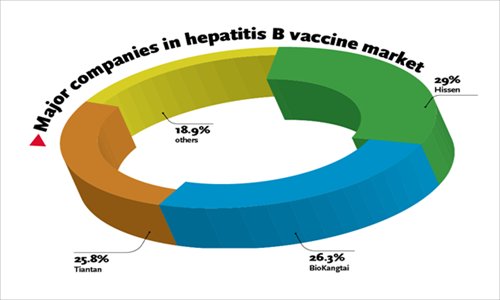
The three companies have a share of more than 80 percent of China's hepatitis B vaccine market, with Hissen taking 29 percent, BioKangtai holding 26.3 percent, and Tiantan owning 25.8 percent as of October 2013, financial news portal caixin.com reported Tuesday.
Vaccine companies that did not obtain the GMP certificate before the December 31 deadline are banned from manufacturing new products in 2014, the CFDA said. But the firms are still able to sell their existing products, the CFDA noted.
North China Pharmaceutical Group Corp, based in Shijiazhuang, capital of North China's Hebei Province, did obtain the GMP certificate for its hepatitis B vaccine product line, news portal 163.com reported Tuesday.
An unnamed staff member of North China Pharmaceutical, which has a 12 percent share of the country's hepatitis B vaccine market, said that the company spent "a very long time" to prepare for the GMP approval and spent half a year to get the certificate, the report said.
Hissen has already applied for a new GMP certificate, which it might receive in the second half of 2014, but the failure to get one before Tuesday will hurt the company's business, 163.com reported.
Tiantan's hepatitis B factory failed the GMP test, caixin.com reported, and the company is not sure if it will renovate the factory. Tiantan plans to apply for a GMP certificate for its new factory in Beijing's Daxing district in 2014 or 2015, the news portal said.
The three firms' failures to get the certificate before the deadline came against a backdrop of growing concern about domestically produced hepatitis B vaccines. Since early December, more than 10 babies have died after hepatitis B vaccine inoculations, prompting the CFDA to ban the use of vaccines produced by BioKangtai.
Yu Mingde, president of the Chinese Pharmaceutical Enterprises Association, told the Global Times Wednesday that China's hepatitis B vaccine market will face a shortage in 2014.
"There is huge demand for hepatitis B vaccines because the inoculation is required and paid for by the government in China," Yu said. "The three companies' stock will be quickly used up, and China may need to import hepatitis B vaccines in the future."
Song Ruilin, executive president of China Pharmaceutical Industry Research and Development Association, told the Global Times Wednesday that there will not be a serious long-term shortage, because other companies will be able to expand their production and fill the void quickly.
"Although the newly adopted GMP rules will hurt some companies and kick some out of the business, it is a vital step for the country to ensure that its hepatitis B vaccines are safe," Song said. "The country should place the quality of the products as a priority, above the need to fulfill market demand."
Only 60.3 percent out of 1,319 sterile drug producers have obtained a GMP certificate, the CFDA said Tuesday, while noting that products made by the GMP-approved companies will be able to fulfill the market demand.
Posted in: Industries