Raw material supply for COVID-19 vaccines sufficient in China
By Chi Jingyi and Yin Yeping Source: Global Times Published: 2020/8/2 20:03:41

A staff member takes out samples of the COVID-19 inactivated vaccine at a vaccine production plant of China National Pharmaceutical Group (Sinopharm) in Beijing, capital of China, April 11, 2020. (Xinhua/Zhang Yuwei)
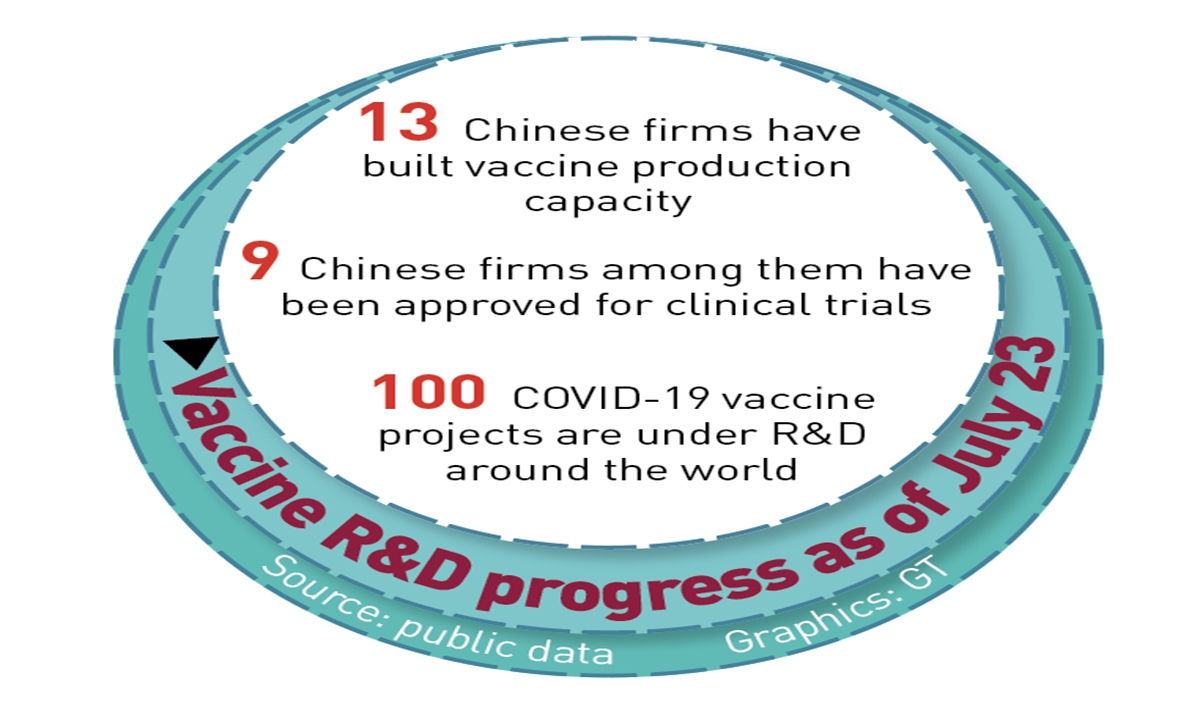
As countries race to promote COVID-19 vaccines, concerns have arisen over the supply of upstream raw materials. Industry analysts said that Chinese manufacturers need not worry as China has a sufficient raw material supply.According to China's Ministry of Industry and Information Technology (MIIT), as of July 23, a total of 13 enterprises in the country have launched construction of assembly lines to produce COVID-19 vaccines, while 9 Chinese enterprises have received regulatory approval to start clinical vaccine trials.
The planned output of vaccines will surpass 200 million doses from Sinopharm China National Biotec Group (CNBG). According to the data published by local media, the output of COVID-19 vaccines next year is likely to hit 500 million doses.
Tao Lina, a Shanghai-based vaccine expert, said that China is fully capable to supply the raw materials needed to produce COVID-19 vaccines, if they are approved by the regulator.
"The production of a COVID-19 vaccine requires the inactivated virus to be cultured in specific cells, for example, chicken embryos, which is the same as the cells used in rabies vaccine, as the two inactivated vaccines have the same production processes and technology," Tao told the Global Times Sunday.
Data from the National Institute for Food and Drug Control showed that 58.83 million rabies vaccines were issued in China in 2019. By the first half of 2020, the total number of issued rabies vaccines in China was 34.17 million, with a year-on-year increase of 27.7 percent.
The production capacity of rabies vaccines can be transferred to that of COVID-19 vaccines, because "the production of inactivated COVID-19 vaccines use much the same technology," Tao said.
The latest example was seen in July at the COVID-19 vaccine production base in East China's Zhejiang Province, which is expected to be put into use in March. The production base will produce COVID-19 and rabies vaccines, according to the Administrative Committee of Ningbo Free Trade Zone.
"There is no need to worry about raw materials such as proteases as they are used in small amounts in producing COVID-19 vaccines," Tao added.
Graphics: GT
In a country such as China that has 1.4 billion people, if the raw materials needed for vaccines rely on imports, it will entail some hidden risks, but the problem has been addressed long ago, Tian Hongjian, a technician with China Medicinal Biotech Association, told the Global Times Sunday.
China has a huge market and at the same time is a big producer of vaccines. The equipment needed to produce COVID-19 vaccines is available, and some production lines are already in place, Tian added.
Yang Xiaoming, chairman of CNBG, said that the inventory of the inactivated coronavirus vaccine in the company has reached 4 million doses, and therefore can quickly meet the Chinese market's demand once the vaccine is approved by the regulator, local media reported Thursday.
Vaccine vials are another concern for many vaccine manufacturers.
An employee of Shandong Pharmaceutical Glass Co. Ltd, who asked not to be named, told the Global Times Sunday that the enterprise alone can produce sufficient vaccine vials.
The A-share listed company is a major vaccine packaging manufacturer in China. According to corporate database Tianyancha, there are more than 60 vaccine-related packaging manufacturers in China.
According to incomplete statistics, the annual output of vaccine vials can reach more than 8 billion, meeting the demand of the coronavirus vaccine production, said the China Association for Vaccines on May 17.
"Currently, most domestic vaccine vials use low-borate borosilicate glass: the price is affordable compared to imported vaccine vials that use borosilicate glass," the employee said, adding that Shandong Pharmaceutical Glass has developed borosilicate glass for high-end consumers.
"However, domestic vaccine vials may not be of that high quality as imported ones in compatibility - and prolonged exposure might affect the quality of the contained vaccine. The coronavirus vaccine will be used as soon as it is produced, so there should be no problem if domestic vaccine vials are used," Wang Xuegong, deputy director of the China Pharmaceutical Enterprises Association, told the Global Times Sunday.
There are more than 100 novel coronavirus vaccine projects under development worldwide, according to the WHO.
Earlier, China has promised that the Chinese-made COVID-19 vaccines will be made available to the entire world.
Newspaper headline: Global concerns rise on vaccine production
Posted in: INDUSTRIES,BIZ FOCUS