Chinese medicine regulator to review EdiGene’s therapy for blood disorders
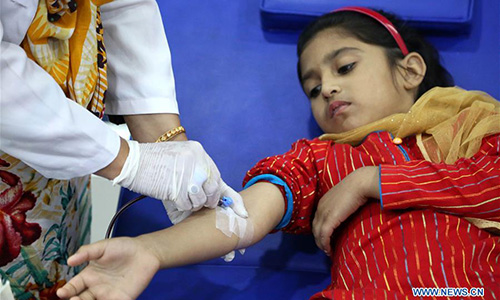
Pakistani patient suffering from thalassemia receives blood at a medical center on the World Thalassemia Day in Islamabad, Pakistan, on May 8, 2018. Thalassemia, also called Mediterranean anemia, is an inherited and non-infectious blood disorder. (Xinhua/Ahmad Kamal)
The China National Medical Products Administration (NMPA) will review biotech company EdiGene's investigational new drug (IND) application for ET-01, an investigational gene-editing therapy for patients with transfusion-dependent beta-thalassemia (an inherited blood disorder), according to the company's website.
The trial of ET-01 is expected to assess its safety and efficacy in transfusion-dependent beta-thalassemia patients. In China, it is estimated that there are 30 million thalassemia gene carriers, and over 300,000 patients with thalassemia major or thalassemia intermediate. Serious unmet medical needs remain for transfusion-dependent beta-thalassemia patients today.
"We are happy to achieve this important milestone and bring ET-01 closer to clinical-use stage," said Dong Wei, CEO of EdiGene. "We are committed to translating cutting-edge gene-editing technologies into transformative therapies so as to bring patients better choices, and for some, a potential one-time cure. We look forward to receiving approval from NMPA and initiating ET-01 clinical studies in the near future," he added.
ET-01 is an investigational gene-edited hematopoietic stem cell therapy for transfusion dependent beta-thalassemia patients.
EdiGene is a biotech company developing genome editing technologies to accelerate drug discovery and develop novel therapeutics for a wide range of illnesses.
Global Times