Joint vaccine trial 'not terminated'; Chinese virologists worry data safety in Canada
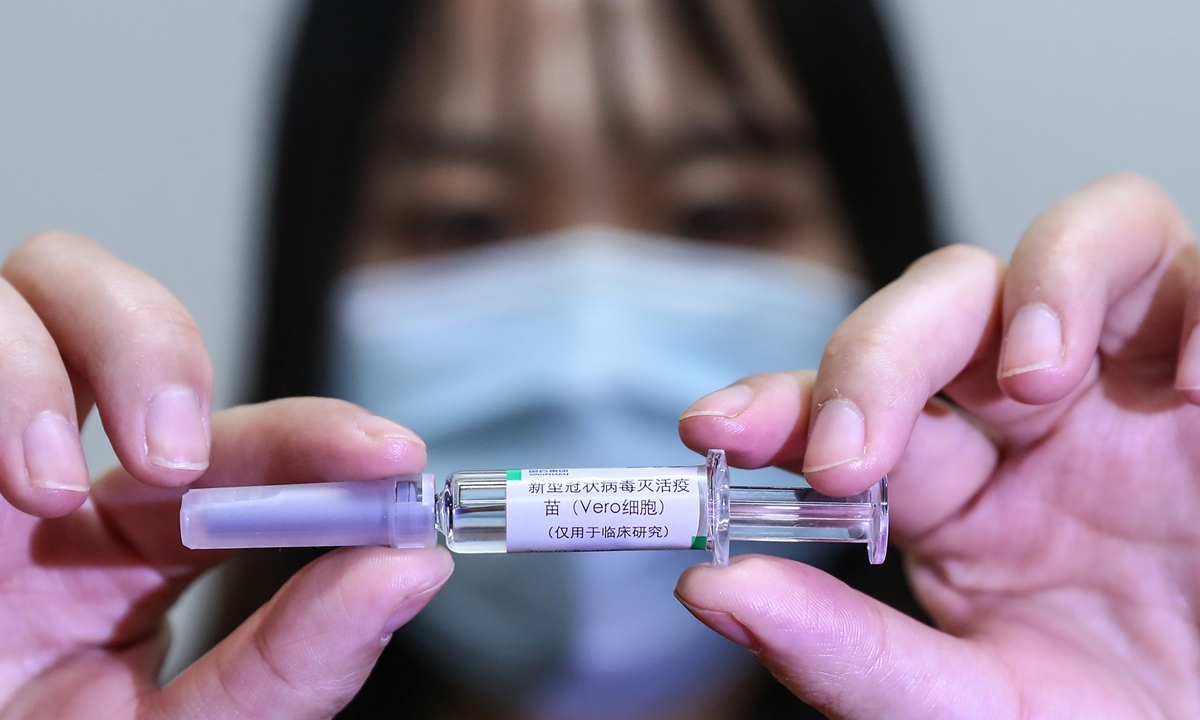
A medical worker shows the inactivated COVID-19 vaccine candidate developed by SinoPharm at the company's vaccine candidate production plant on April 10. Photo: Xinhua
Chinese vaccine developer CanSino Biologics said on Thursday that its collaboration with a Canadian research team on late-stage clinical trials of a COVID-19 vaccine candidate "has not been terminated," refuting reports by Canadian media which said the cooperation was called off for rising tensions between the two countries.CanSino sent an English clarification announcement to the Global Times on Thursday night, in which it said the collaboration with the National Research Council of Canada (NRC) has not been terminated. None of the management of the company has accepted any interview in relation to the clinical trials for Ad5-nCoV in Canada.
CanSino also said in the Thursday announcement that it noticed misleading media reports related to the clinical trials for the vaccine candidate in Canada.
Canadian media CBC News on Wednesday reported that the cooperation of the two sides which was announced in May was "abandoned amid rising tensions between the two countries." The report quoted an emailed statement from the NRC as saying that the Chinese Customs had not approved the vaccine candidate, named Ad5-nCoV, to be sent to Canada.
CanSino said on August 18 that the Phase III clinical trials with Canada had not yet started.
Canadian media even called the delayed shipments as an "apparent retaliation" from China for the arrest of Huawei CFO Meng Wanzhou.
Aside from saying it is currently driving the international multi-center phase III clinical trial for Ad5-nCoV with several countries, CanSino did not provide further details on the cooperation with the NRC in the announcement and NRC has not responded to a Global Times request as of press time.
However, some virologists reached by the Global Times believe that scientific, elements may have played a role in the twists and turns of the collaboration, though Canada's damage to legal and diplomatic relations may also be a factor.
The types of COVID-19 that have spread in North America are different from those in Asia or Europe, making Canada a less ideal place for clinical trials of this China-based vaccine, said Yang Zhanqiu, a professor at the pathogen biology department of Wuhan University.
"Also, the number of infected patients in Canada is fewer than many other countries," Yang told the Global Times on Thursday, saying that made Canada a less favorable destination for clinical trials of a recombinant vaccine that requires a large group of volunteers facing a higher risk of infection.
Canada has reported more than 128,000 cases of COVID19 so far, with a downward trend since early May.
Another Beijing-based virologist who asked not to be named said that property rights disputes or other legal concerns may also put obstacles to the cooperation, as media reported the Ad5-nCoV used a piece of Canadian technology - a cell line modified by the NRC - which the NRC had provided to CanSino for use in Ebola vaccine R&D in 2014 and coronavirus vaccine research in 2020.
The twists of the cooperation deal may have also reflected China's possible doubts about the safety of conducting trials in Canada over concerns Chinese vaccine R&D data may be leaked under outside pressure. Canada has already betrayed Chinese companies due to US pressure, Tao Lina, a Shanghai-based immunological expert, told the Global Times on Thursday.
China, along with other countries such as Russia and Saudi Arabia, has made progress in Phase III trials cooperation of the COVID-19 vaccine, Tao said.
Volunteers of joint China-Russia trials on the same vaccine candidate are scheduled to be all vaccinated by the end of September, and the results will be released by late fall, the Global Times learned on Sunday from Petrovax, a leading Russian pharmaceutical products developer.
"China has long rejected vaccine nationalism or politicization of coronavirus vaccines, as can be seen in its extensive cooperation with other countries," Tao added.