Photo: VCG
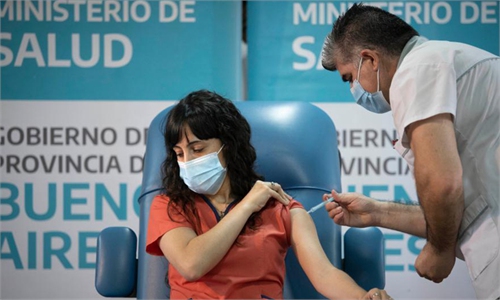
Like many pinning hopes of ending the pandemic on vaccines, European countries have also bet their economic recovery on a cure for the virus, which is also why the EU organized the joint procurement program earlier in 2020 to ensure the vaccine availability for all of its member states. Yet, the slow progress underlines that this is actually a test of a country's manufacturing and supply chain strength.While the EU started a mass COVID-19 vaccination drive in late December, the uptake pace has been alarmingly slow in some countries.
In particular, the French government has come under criticism for lagging behind its EU neighbors in rolling out vaccinations. As of 5 pm Tuesday, France vaccinated more than 5,000 people in one day, and its Health Minister Olivier Veran said his government would soon catch up with the likes of Germany. More than 200,000 people were vaccinated in Germany in the first week of the vaccination campaign. France's accelerated vaccination effort is laudable, given the fact that only 500 people had been vaccinated in the country in the first week of the country's rollout of the Pfizer-BioNTech vaccine, which started on December 27.
France is one of the EU countries that are struggling to address the slow pace of COVID-19 vaccination, another worrying development that goes against original expectations. This is because when scientists and researchers in the West were working around the clock to develop the COVID-19 vaccine, no one could have expected that delivering vaccination would become a major bottleneck.
Given the high level of public skepticism about the vaccine, it seems kind of understandable for the French government to set up strict guidelines for a pre-vaccination consultation and getting consent. But the only problem is that it takes more time, putting an even greater burden on the logistics, which could have already been strained due to the high requirements of vaccine storage.
The Pfizer-BioNTech vaccine, which must be stored at -70 C, has very strict requirements for transportation and time of use. Assuming that the Oxford-AstraZeneca vaccine would be ready first, the Netherlands was not even prepared to receive the Pfizer-BioNTech vaccine, and its vaccination work won't start until Wednesday, 10 days after their EU neighbors.
A series of issues exposed during the vaccination process which have highlighted a weak manufacturing industry across some European countries. The inefficient logistics system and supply chain, plus the time needed to procure storage equipment and materials like freezers from other countries, may become a new source of uncertainty for the future vaccination drive.
To a certain extent, resource integration and deployment ability involved in getting the mass public vaccinated points to whether the external environment could provide the necessary support that a country's manufacturing development needs. And in that sense, the vaccination issue in some European countries may reflect the sign of a manufacturing base hollowing out.