Rare cases of blood clots after taking J&J jabs raise global concern, Chinese experts urge enhanced info sharing
Rare blood clots after taking J&J jabs 'no hindrance' to global efforts
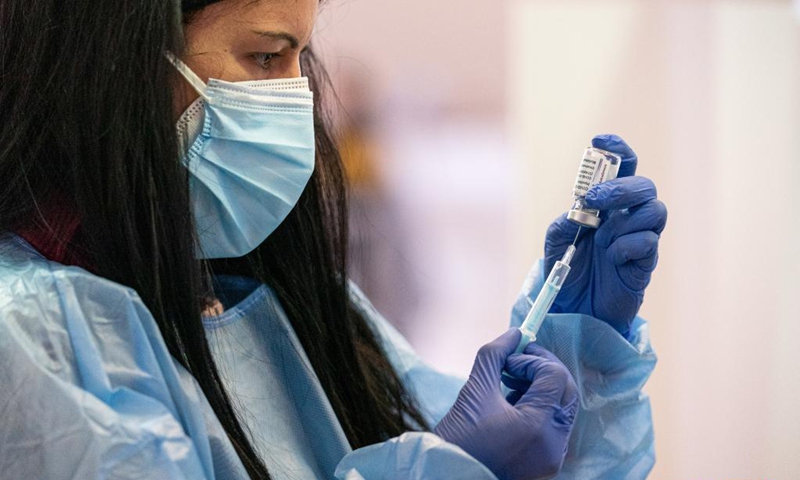
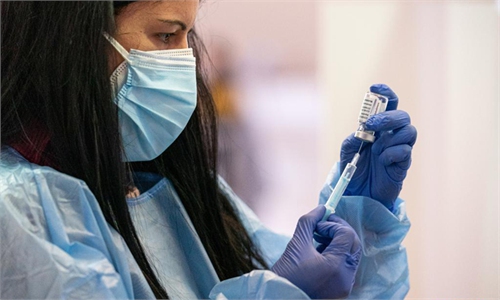
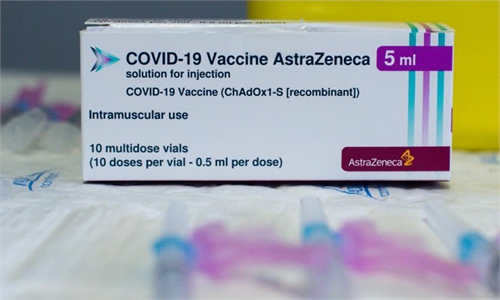
A health worker prepares a dose of AstraZeneca COVID-19 vaccine at a vaccination site in Cornella, Spain, on Wednesday. The European Medicines Agency (EMA) confirmed on Wednesday that the occurrence of blood clots with low blood platelets are strongly associated with the administration of AstraZeneca COVID-19 vaccine, but should be still listed as very rare side effects. Photo: Xinhua
Chinese experts have called on the international community to enhance information sharing on vaccine-related studies amid rising safety concerns as three sites in the US halted emergency use of Johnson & Johnson (J&J)'s adenovirus vaccine after a number of adverse reaction cases including blood clot were reported following the jabs.However, the global vaccination drive should not be hindered, as no causal connection has been established between the vaccines and serious adverse effects, they noted, while calling for more thorough follow-up examinations.
Public concerns peaked as the US state of Georgia halted the use of J&J's vaccine after eight people suffered adverse reactions to the shot, the third site to have shut down the vaccine following North Carolina and Colorado, which reported 18 and 11 adverse reactions ranging from dizziness to nausea and fainting, US-based CBS News reported on Saturday local time.
Following the incidents, shipments of J&J's vaccines, which were previously touted by some health experts as a promising replacement for the AstraZeneca jab, were scaled back dramatically by 86 percent in the coming week, the report said. Several densely populated cities in the US, including those in the states of Massachusetts and New York, already announced a major decrease in the number of J&J doses to be administered next week, according to local media.
Meanwhile in Germany, some 42 suspected cases of rare blood clots were reported after the AstraZeneca jab was administered, as well as 23 cases with a plunge of platelets in the blood, the Paul Ehrlich Institute said on Friday.
Tao Lina, a Shanghai-based vaccine expert, told the Global Times on Sunday that the possibility of the vaccines causing rare clotting syndromes cannot be ruled out, as data has pointed to a higher occurrence rate after taking the jabs.
But it should not become a cause for concern, noted experts, as reported incidents are extremely rare cases that account for one in a million, and "the pace of the global vaccination rollout should not be affected as the benefits of taking the shots still outweigh its risks," Feng Duojia, president of the China Vaccine Industry Association, told the Global Times on Sunday.
Responding to growing safety concerns, France decided on Friday to give shots from a different producer, either Pfizer-BioNTech or Moderna, as their second course for people under the age of 55 who had already been given the AstraZeneca as their first dose, France24 reported. Some 533,000 people are affected by the decision, the French national health authority said.
For people receiving mixed doses, Tao said more observations are needed. Tao believes that efficacy could still be ensured, or "produce an even stronger protection against the virus."
Chinese health experts also called for closer collaboration across countries on related studies, as China's CanSino vaccine is an adenovirus vector shot, although no serious side effects have been observed so far.
"More extensive recognition of COVID-19 vaccines can only be achieved if world scientists work together and provide references for each other," Feng said.
The EU drug regulator said on Friday it is reviewing cases of rare blood clots in four people in the US, one of whom died, who received the J&J vaccine, Reuters reported. The agency previously listed the occurrence of blood clots as very rare side effects of AstraZeneca COVID-19 vaccine, and it is now reviewing reports of capillary leak syndrome in people given its shots.