What potential risks lay ahead for China’s COVID-19 vaccine export?

The cargo shipment of COVID-19 vaccines produced by the Chinese company Sinovac Biotech is being dispatched at the Cumbica airport in Guarulhos, Sao Paulo state, Brazil, on July 20. Photo: AFP
Brazil's sudden suspension of Chinese COVID-19 vaccine trials on November 9 raised concerns about obstacles China faces in exporting its vaccines. Although clinical trials of the vaccine soon resumed in Brazil, political wrangling behind multi-layered stakeholders and commercial interests in host countries pose increasing potential risks to Chinese vaccines' distribution abroad.
Brazil is not the only country where China's vaccine exports have encountered setbacks and potential resistance. Chinese-developed vaccines have also been blocked by certain political forces in some South Asian countries, an industry insider told the Global Times.
Pledging to send vaccine supplies to a growing number of countries, China is seen by many as the global leader dealing with pandemic -- a void left by the US. However, China still may struggle to gain recognition amid misinterpretations around its vaccines like its exported facemasks and ventilators in the early days of the pandemic, experts warned.
The political risk is high since a vaccine is a game changer to the pandemic, while at the same time a medical product with side-effects is rarely 100 percent effective, said Dr Jennifer Bouey, an epidemiologist and senior policy researcher at the US-based think tank RAND Corporation, in a recent interview with the Global Times.
Politicized vaccine
China on Tuesday reiterated its call for closer vaccine cooperation with the BRICS countries, including Brazil, following the storm over the past week that halted clinical trials of a Chinese-produced vaccine in Brazil.
The halt was widely speculated as being the latest flashpoint in the ongoing spat between political rivals Brazilian President Jair Bolsonaro and São Paulo Governor João Doria over the handling of the coronavirus that killed more than 167,000 people in Brazil.
The halt raised wide suspicion Anvisa made the decision under the pressure of the president who has repeatedly railed against a Chinese-developed vaccine and the measures China took against the COVID-19. Antônio Barra, director of Anvisa, told local media there was "unpleasant and bothersome" political pressure, but that it did not affect the regulator's work, Financial Times reported.
While Bolsonaro has expressed Brazilians should not be "guinea pigs" for the Chinese-produced vaccine, Doria promoted the state-level deal with China to import 120,000 doses of CoronaVac scheduled on November 20 upon final approval of Anvisa.

An engineer walks out a storeroom at the general laboratory during a media tour of a new factory built to produce a COVID-19 vaccine at SinoVac in Beijing on September 24. Photo: AFP
Whereas Bolsonaro is looking to a COVID-19 vaccine developed by the University of Oxford and AstraZeneaca. He issued a decree in August to purchase 100 million doses. But the vaccine candidate was temporarily halted in September after a participant experienced an unexplained illness. But Bolsonaro has ignored public concerns over the vaccine candidate's safety. Some observers thus see the row in Brazil as a vaccine race between China and the West.
Setbacks around Chinese-developed vaccines like what had happened in Brazil are not unique. The Global Times has learned the late-stage trials of CoronaVac in Bangladesh were thwarted by high-level political forces and even faced obstructions in the early stage of trials initiation. Bangladesh finally decided to not co-fund a late-stage domestic trial of a potential coronavirus vaccine CoronaVac, Reuters reported on October 13.
The cooperation between Chinese vaccine developer CanSino Biologics and the National Research Council of Canada (NRC) also seemed to experience hiccups, with media claiming that the joint vaccine trials between the two were suspended in August due to unsuccessful approval for transnational transportation, though CanSino later released a statement denying "termination" of the cooperation.
Multiple risks
Misrepresentation of the trials suspension in Brazil brought false claims about Chinese-developed vaccines across Brazilian social media, with an unidentified man even claiming that China has injected particles of the COVID-19 disease into the face masks it exports to Brazil, media reported.
The conspiracy theories and political battles surrounding China's vaccines have raised concerns about the political risks of exporting vaccines, as any failure in clinical trials, transportation, or vaccination could be maliciously interpreted.
China's vaccine distribution has focused on its domestic market and developing countries. Whether the local public will accept the Chinese vaccine or not depends on domestic politics, said Dr Jennifer Bouey in a recent media interview.
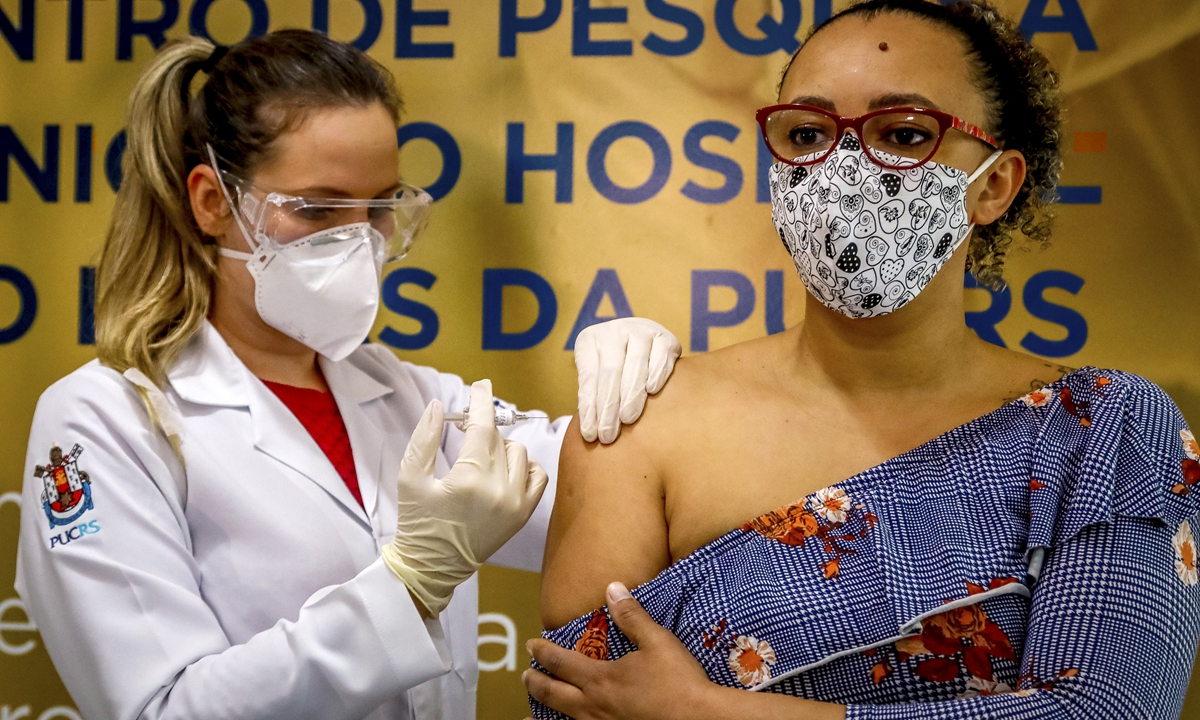
Health worker and volunteer Fabiana Souza receives a COVID-19 vaccine produced by Chinese company SinoVac Biotech at the Sao Lucas Hospital, in Porto Alegre, southern Brazil on August 8. Photo: AFP
"A vaccine roll-out plan, therefore, will depend on not only the government/party currently in power, but also on opinions of the other domestic political stakeholders. China's vaccine assistance program will benefit from taking into consideration messaging to all potential political influencers in an aid-recipient country," Bouey told the Global Times.
Peking University professor Zhang Yiwu told the Global Times that international rivalry for vaccine R&D indeed exists in the short term. Whoever develops the vaccine first will have more advantages, and the West is worried those advantages will be claimed by China, said Zhang.
"In addition to scientific achievements and financial gains, whoever first grasps the vaccine will have the key to restart the economy and society amid such a difficult time," Zhang suggested.
As global vaccination against the coronavirus is likely to be the largest in human history, possibly reaching several billion people, it will likely involve various legal disputes and claims, a representative of China's leading vaccine producer who requires anonymity told the Global Times.
The COVID-19 vaccine distribution will also encounter unprecedented logistical challenges. "It will need to reach nearly 200 countries in a relatively short timeframe and will need to move in a safe and secure manner to ensure full compliance with cool chain temperature requirements," Glyn Hughes, head of cargo at the International Air Transport Association, told the Global Times in a previous interview.
Other difficulties lie in shortages of supporting facilities and high cross-border transport costs. As the order for transnational transportation of vaccines increases, international freight market prices may surge when vaccines start to be shipped. Customs clearance approvals are either stagnant or slowing down in many countries, which pose greater risks on vaccine's storage in ports, Chen Yong, manager of the Lengwang Technology Co., which provides cold chain solutions for traditional medical products logistics, told the Global Times.
Photo:VCG
Scientific responses
A successful transnational transportation of COVID-19 vaccines requires thorough negotiations between China and the vaccine recipient countries on the potential risks and insurance cover plans in advance, and preparations for the worst, industry insiders told the Global Times.
"This requires Chinese vaccine manufacturers and overseas partnered institutions to closely monitor the feedback of a vaccinated public along the whole process of mass inoculation, in case of large-scale severe adverse events like the flu vaccine death incidents in South Korea," Tao Lina, a Shanghai-based vaccine expert, told the Global Times.
Countries with different medicine regulatory systems and health financing mechanisms can work with China, the US, other countries, and international organizations like WHO and COVAX to map the supply allocation, according to Bouey.
"A successful vaccine will soon become an important diplomatic asset for China, and it will help improve China's global image if it leads in vaccine R&D and provides it to more developing countries," Wu Xinbo, dean of the Institute of International Studies at Fudan University in Shanghai, suggested.
China has bet on biotech as its ten-year national plan will propel its biomedical and medical device manufacturing industry. Biotech growth is also part of the Made in China 2025 plan along with its 5G technology and chips. Developing a vaccine for the world would also elevate China's status as a competitive global scientific and medical power.
Newspaper headline: Injecting hysteria
- China to provide COVID-19 vaccines to Africa to support virus control: FM
- Advantages of China’s COVID-19 inactivated vaccines in the world vaccine competition
- Chinese vaccines also very effective: China’s CDC director
- China's 1st COVID-19 recombinant subunit vaccines begin phase-III trials domestically