Sinopharm eyes bringing COVID-19 vaccine to market, says clinical trial results in various countries ‘good’
By Hu Yuwei and Leng Shumei Source: Global Times Published: 2020/11/25 12:49:23
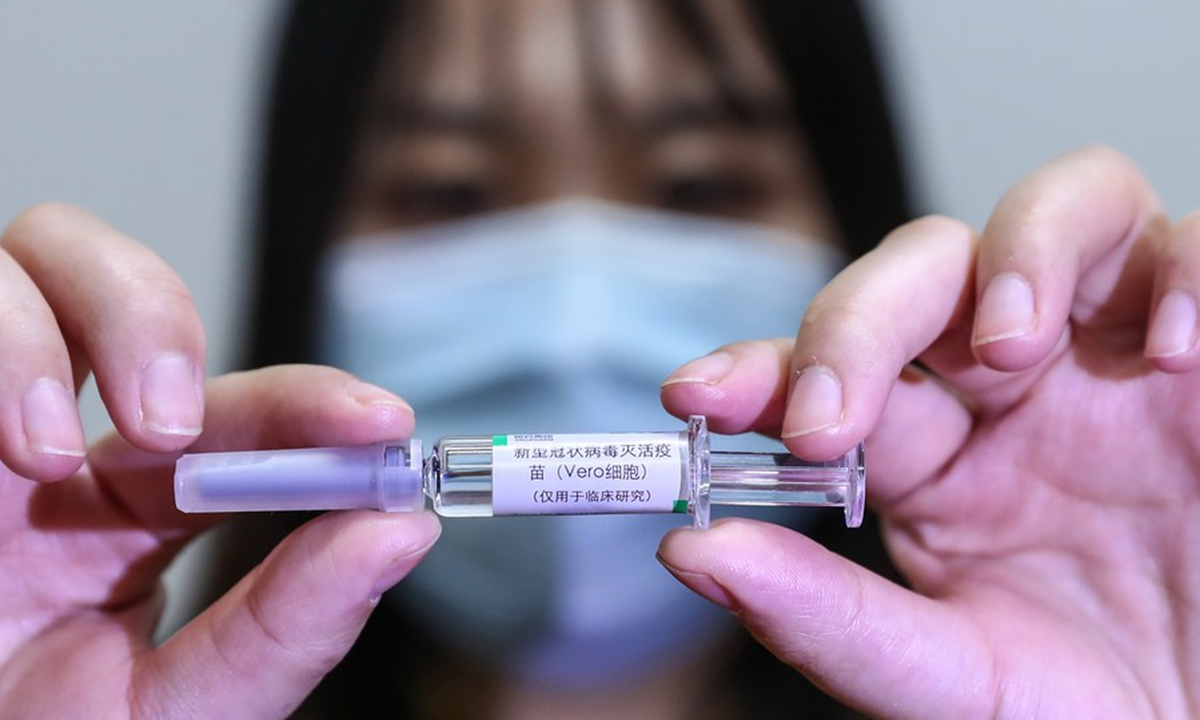
A staff member displays a sample of the COVID-19 inactivated vaccine at a vaccine production plant of China National Pharmaceutical Group Co., Ltd. (Sinopharm) in Beijing, capital of China, April 10, 2020. (Xinhua/Zhang Yuwei)
China's leading vaccine producer Sinopharm announced Wednesday that it has submitted an application to Chinese authorities for marketization of its COVID-19 vaccine.
A vice-general manager named Shi Shengyi made the announcement.
A representative of Sinopharm told the Global Times on Wednesday that Sinopharm had collected data from major countries carrying out its vaccine clinical trials such as UAE. Results are expected to be good but it is up to Chinese authorities to make the decision as the authorities have strict review standards.
Sinopharm previously told the Global Times that it had reported the phase-III clinical data to China's State Food and Drug Administration and was in the process of giving more detailed data, as requested.
The vaccine maker says it is in the final stage before marketization, and is now making approval for the marketization of the vaccine its top priority. It will be possible to release the phase three data in academic journals after the approval.
“Our data is evaluated by relevant departments following protocols even stricter than in some Western countries, and we are in close communication with the WHO," a representative of Sinopharm told the Global Times previously.
Global Times
Posted in: SOCIETY