China is preparing for COVID-19 vaccine mass production: official
By Leng Shumei and Hu Yuwei Source: Global Times Published: 2020/12/16 21:53:40 Last Updated: 2020/12/17 1:32:07
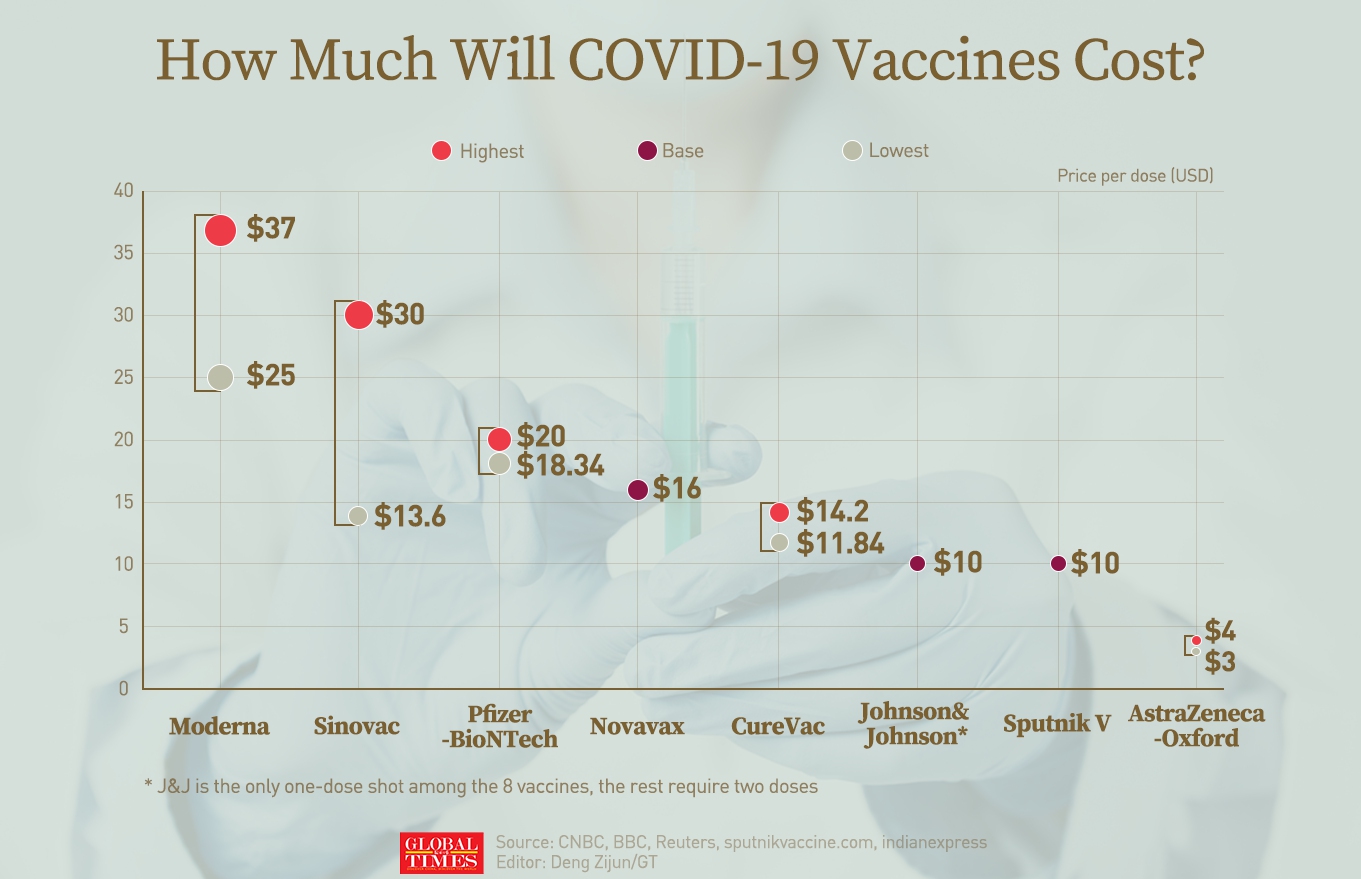
How much will COVID-19 vaccines cost? Infographic: GT
China is preparing for mass production of COVID-19 vaccine, said national health authority on Wednesday.
Zheng Zhongwei, director of the Development Center for Medical Science and Technology of the National Health Commission (NHC), told the Xinhua News Agency that related institutes are promoting phase three clinical trials on domestic developed vaccine candidates, strictly following laws and regulations and international standards to ensure the candidates' safety and efficacy.
"Vaccine research and development has entered the final stretch. Although China is among the top tier in the world in vaccine development, it will not jump the gun just to become the first," Zheng said.
There are a lot of criteria to evaluate a vaccine, among which the most important are safety, efficacy, accessibility and affordability, Zheng said.
He noted that China is preparing for mass production of the vaccine.
According to Zheng, 15 vaccine candidates developed in China have entered clinical trials with five of them in phase three.
As China has put the epidemic under control, the phase three clinical trials have to be conducted overseas, facing some difficulties and challenges, Zheng said.
More locations in China are preparing for mass vaccination against the novel coronavirus as vaccines are likely to be available by the end of this year.
The Global Times learned from a source that the national disease control and prevention system held a video conference on Wednesday to train employees across the country on the work of vaccination, including an introduction to the COVID-19 vaccines, how they are to be administered, how to deal with abnormal reactions and requirements on circulation management.
The information indicated that some COVID-19 vaccines are very likely to be approved by the national authority and provided to the public soon, Tao Lina, a Shanghai-based expert on vaccines and a former Shanghai disease prevention and control employee, told the Global Times on Wednesday.
Chinese authorities have said on many occasions that domestically made vaccines are likely to be available by the end of this year.
But Tao predicted that the vaccination would not be so "mass" or carried out very "swiftly," as many Chinese people live in places where the epidemic has been brought under control, and they may think it is not necessary to get vaccinated.
"At the beginning, the priority will be given to people at high risk like medical staff and people in the cold-chain industry, but the public may also get vaccinated if they register," Tao predicted.
Sinopharm Holdings, affiliated with China's leading vaccine producer Sinopharm Group, has started a large-scale exercise across China to test its capacity and capability for mass distribution of vaccines, the Global Times learned.
Analysts said logistical practices should be strengthened in areas with sporadic COVID-19 outbreaks such as Northwest China's Xinjiang Uygur Autonomous Region, Southwest China's Sichuan Province and Northeast China's Heilongjiang Province, which will possibly be the first to receive the vaccine.
Tangyuan county in Heilongjiang on Tuesday opened registration for the public to sign up for two doses of the COVID-19 vaccines for 420 yuan ($65) in total, becoming the first region in China to officially open early vaccine accessibility to residents.
Local residents can voluntarily get vaccinated at designated medical institutions at charge of 420 yuan for two doses per person, including the cost of syringes, injections and insurance, according to an official statement from the county government on Tuesday.
A staff member of the county's epidemic prevention and control office told the Global Times on Wednesday that each local resident is eligible to sign up for the vaccination. But the first doses have yet to arrive, and the registration is underway to gauge the number of people willing to receive doses.
About 250,000 residents live in the Tangyuan county.
Southwest China's Sichuan Province has also begun coronavirus vaccine emergency use for 12 types of high-risk groups - about 2 million people - with inoculations scheduled to be completed by the end of the year, media reported.
It was reported that inactivated COVID-19 vaccines were the main vaccines to be administered in Sichuan. The vaccine costs 200 yuan per dose, the same as the one used in Zhejiang Province, which was launched in October.
East China's Jiangsu becomes the latest province to purchase two types of domestically developed inactivated vaccines for emergency use at this wholesale price. According to a public document posted on the official website on Tuesday, the Jiangsu provincial government signed with two leading Chinese COVID-19 vaccine producers — Sinopharm and Sinovac — at the wholesale price of 200 yuan ($30.57) per dose.
Posted in: SOCIETY