Chinese-developed inactivated vaccine has low rate of adverse reactions
By Hu Yuwei and Leng Shumei Source: Global Times Published: 2020/8/14 23:18:55
vaccine Photo:VCG
A Chinese-developed inactivated COVID-19 vaccine on Thursday revealed a low rate of adverse reactions for patients in phase one and two clinical trials while also demonstrating immunogenicity results. The interim analysis of two randomized clinical trials suggests a relatively better safety profile compared with other kinds of the same type of vaccine, researchers said in the latest report.
The report, published in peer-reviewed medical journal The Journal of the American Medical Association on Thursday, is the first formal clinical trial data for a COVID-19 inactivated vaccine, the vaccine producer China National Pharmaceutical Group (Sinopharm) told the Global Times in a statement on Friday.
Within seven days of injection, adverse reactions were reported by 48 (15.0 percent) of 320 participants in the trials, the report showed. All adverse reactions were mild, transient, and self-limiting, and did not require any treatment. No other adverse reactions were reported between days 8 and 28 after injection.
China's coronavirus vaccine R&D has always put safety as a top priority, and it will make sure all three phases of clinical trials are completed before formal approval, Tao Lina, a Shanghai-based vaccine expert, told the Global Times.
China is one of the countries that has unreservedly disclosed most data and results surrounding the safety and immune response of vaccine candidates, Tao said.
So far, at least three Chinese primary vaccine producers have delivered precise and detailed data for phase one and two trials.
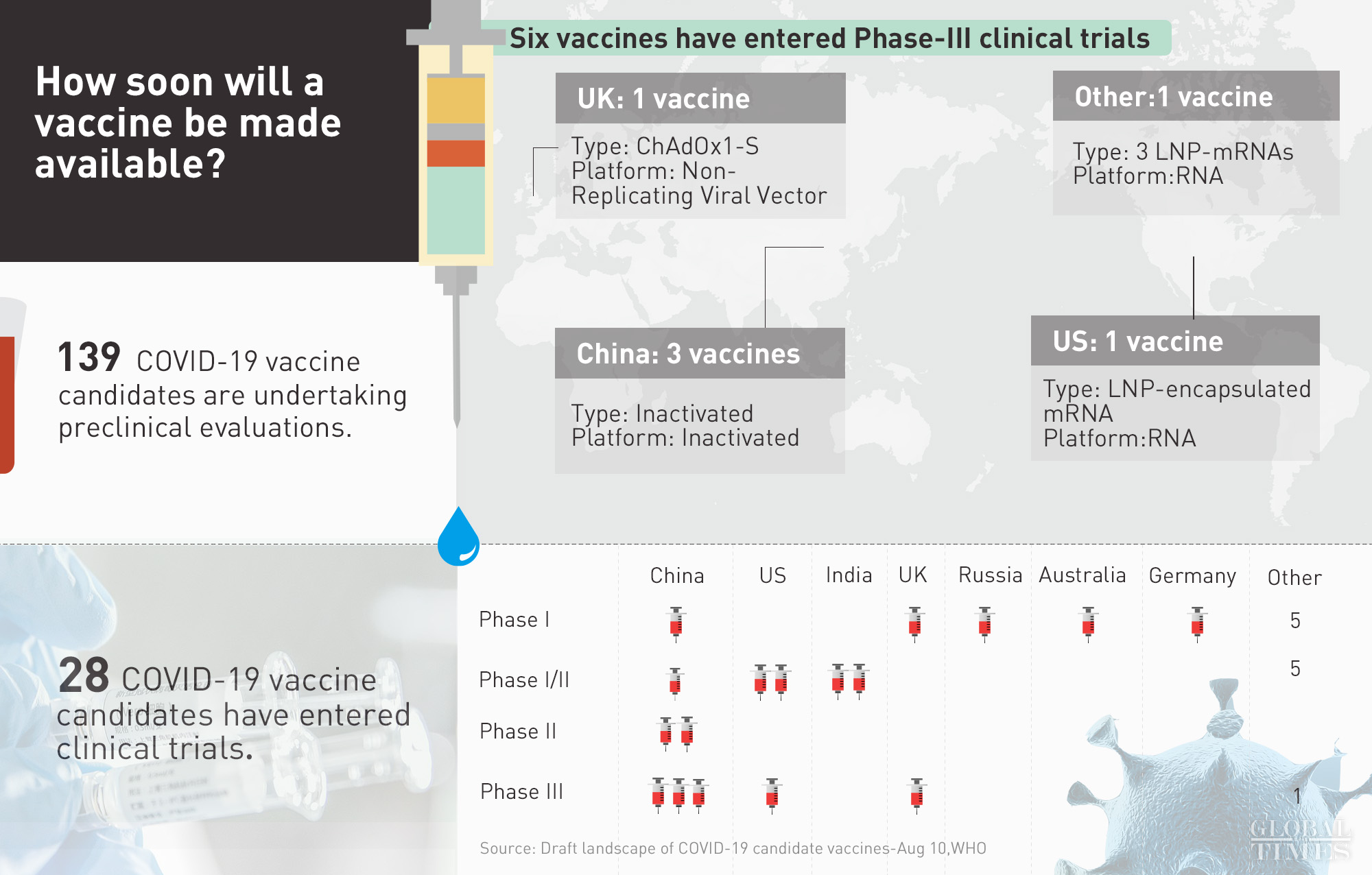
Infographic: Global Times
The vaccine developed by Sinopharm is now undertaking its phase three trials in the UAE with more than 15,000 volunteers participating, while the early-stage trials for some remaining groups are still going ahead as planned, Sinopharm told the Global Times.
The optimal interval between injections and times for booster injections of the inactivated vaccine remains unclear, and the full analysis of the trial data with extended follow-up and other intervention groups is needed, according to the report.
Sinopharm told the Global Times that the results of phase one and two trials for another inactivated candidate they developed will be revealed in a few days.
The company estimated that an inactivated COVID-19 vaccine will be available on the market at the end of this year or early in 2021. The combined annual production capacity of the group will exceed 200 million doses when mass production is realized, to ensure the accessibility of COVID-19 vaccines, according to media reports.
RELATED ARTICLES:
Posted in: SOCIETY