China’s CanSino launches Phase III clinical trial of COVID-19 vaccine in Mexico, 1st group gets vaccinated
Source: Global Times Published: 2020/11/7 22:26:34
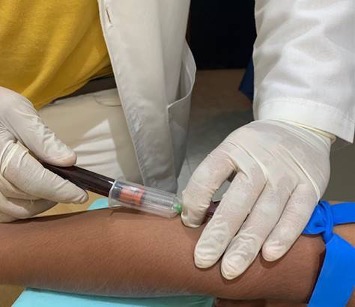
The first group of subjects in Mexico got vaccinated on Friday.
One of China’s vaccine developers announced on Saturday that it has launched the Phase III clinical trial for its COVID-19 vaccine in Mexico and has successfully vaccinated the first group of subjects.
CanSinoBIO, the Chinese pharmaceutical company, told the Global Times on Saturday that it has initiated the Phase III clinical trial for a recombinant COVID-19 vaccine (Ad5-nCoV) it developed in Mexico.
The study was approved by the Mexican authority in October. The trial aims to recruit a total number of 15,000 subjects to further demonstrate the efficacy of the candidate.
In October, CanSinoBIO also signed an advance purchase agreement with Mexico’s government to supply 35 million doses of COVID-19 vaccine to the Mexicans in need.
Xuefeng Yu, chairman and CEO of CanSinoBIO, said, “Launching the clinical study of Ad5-nCoV in Mexico represents another milestone of CanSinoBIO,” and that the company is delighted to collaborate with Mexican local company EPIC to make this initial clinical study in Mexico possible.
Ad5-nCoV is currently undergoing phase III clinical development in both Russia and Pakistan.
Petrovax, CanSinoBIO’s partner in Russia, told the Global Times on Saturday that they have completed the vaccination of all volunteers within the local phase III clinical trial of the Ad5-nCov vaccine candidate, developed by CanSinoBIO.
Following vaccination and examination of volunteers during the first 28 days, the analytical part of the study will be launched with the primary processing of the results. The preliminary results of the study will be received by the end of 2020, according to Petrovax.
Brazilian health regulator Anvisa announced authorization for Sao Paulo's Butantan Institute biomedical center to import 6 million doses of a COVID-19 vaccine developed by Chinese company Sinovac Biotech, which is undergoing final clinical trials in Sao Paulo state, Reuters reported in October.
Vaccine experts told the Global Times previously that China is taking the lead in COVID-19 vaccine research, with China-developed candidates proving their safety and efficacy during clinical trials and emergency use on tens of thousands of Chinese people.
Liu Jingzhen, chairman of Sinopharm, another Chinese vaccine developer, said at a conference on Friday that some 100,000 people have been vaccinated with the company's vaccine and have shown no adverse reactions so far. Among those, 56,000 people were inoculated and travelled aboard, and none have been infected with the virus, said Liu.
Global Times
Posted in: SOCIETY