China likely to approve a COVID-19 vaccine by year end to meet the need of epidemic-stricken countries: experts
By Leng Shumei Source: Global Times Published: 2020/11/25 4:17:48
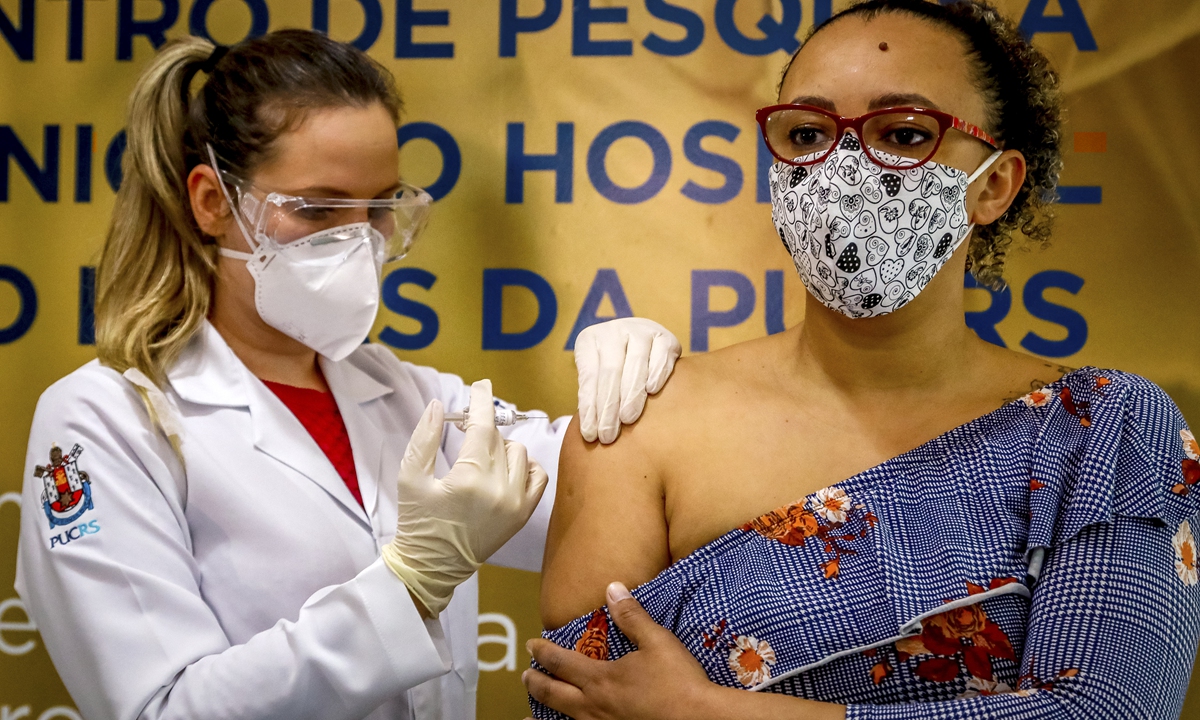
Health worker and volunteer Fabiana Souza receives a COVID-19 vaccine produced by Chinese company SinoVac Biotech at the Sao Lucas Hospital, in Porto Alegre, southern Brazil on August 8. Photo: AFP
As Brazilian health officials expressed hope for China to hasten approval process for a China developed vaccine and more countries are laying out vaccination plan, some experts said that it is possible for China to approve a vaccine by the end of this year to meet the urgent need of some countries severely hit by the epidemic.
Brazil has gathered enough infection data from a late-stage trial of an experimental COVID-19 vaccine developed by China's Sinovac Biotech (SINOVAC) and expects to have interim results on its efficiency in the first week of December based on analysis of the data from an independent committee, Reuters reported Tuesday, citing trial organizers.
João Gabbardo, head of the Sao Paulo's COVID-19 contingency committee, said the same day that he expects China's health regulator to approve SINOVAC's CoronaVac in December, which could hasten approval in Brazil, according to the Reuters report.
SINOVAC confirmed the plan with the Global Times on Tuesday but appears to be more cautious than their Brazilian partners as the company stressed that it is only a preliminary result.
"It not something that could be rushed," Liu Peicheng, a spokesperson from SINOVAC told the Global Times.
According to Liu, the independent committee is constituted of members from international organizations like the World Health Organization and the Performance Assessment Tool for Quality Improvement in Hospitals.
Some Chinese analysts also prefer to stay cautious. They said that they understand Brazil's urgent need for a vaccine given the severe epidemic situation in the country, but they do not think Chinese authorities will hasten approval process at the cost of safety and efficacy.
But some others noted that it is actually hopeful that a vaccine candidate would be approved by Chinese authorities by the end of this year considering the growing need of many foreign countries and China's promise to provide affordable COVID-19 vaccines and drugs for "all people".
According to media reports, Spain will begin a comprehensive coronavirus vaccination program in January and expects to have covered a substantial part of the population within three months.
Russia also announced on Tuesday to kick off massive coronavirus vaccination in 2021.
"Although the epidemic in China is under control, China would very likely hasten vaccine research and development for other countries," Tao Lina, a Shanghai-based expert on vaccine and immunology, told the Global Times.
Foreign countries can decide whether to use China-developed vaccines based on their own situation, but it will enhance their confidence on China-developed vaccines if Chinese authorities approve them first, said Tao.
Tao also said he believed that some Chinese vaccine producers have actually collected enough data that shows positive results.
China's leading vaccine producer Sinopharm previously told the Global Times that it has reported the phase-III clinical data to China's relevant regulators and is improving the data as requested. The vaccine maker, claiming it is in the final stage before marketing, stressed that it is not under pressure to reveal its vaccine data and will do so in a more scientific and controlled way.
Posted in: SOCIETY