SOURCE / INDUSTRIES
China's first portable ECMO system begins registration process, brings hope for domestically-produced machines
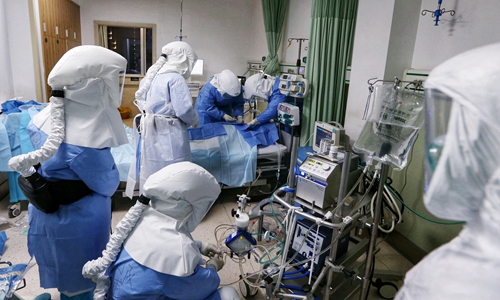
Doctors from East China's Jiangsu Province deployed to Wuhan use ECMO to treat a COVID-19 patient in critical condition at Wuhan First Hospital. Photo: Cui Meng/GT
A medical technology company in East China's Jiangsu Province independently developed China's first domestically-produced portable ECMO system, which has begun the registration process.The Saiteng OASSIST ECMO system was developed by Jiangsu Saiteng Medical Technology Co in Suzhou Industrial Park. The pre-charge amount of the pump head of the system is smaller with more even flow field compared with other ECMO machines, media reports said.
This is also the only domestic ECMO system in China that has begun the registration process.
So far, the OASSIST ECMO system has finished several animal trials successfully.
The system marks an opportunity for China to break free from its long-term dependence on importing these life-saving machines.
"Generally speaking, entering the registration process means there is great hope for the country to manufacture domestically-made ECMO machines in future," Xu Jiarui, director at the research center of a professional sub-device under China Association for Medical Devices Industry, told the Global Times on Sunday.
An abbreviation of Extracorporeal Membrane Oxygenation, ECMO is commonly known as the "artificial lung" and is mainly used to provide continuous external breathing and circulation to patients with severe cardiopulmonary failure.
An ECMO machine costs between 1 and 3.5 million yuan ($140,000 - $500,000) with additional high costs on consumable items. It can cost a patient about 200,000 yuan for only two weeks' treatment in the ICU, the Xinhua News Agency reported citing an expert in April.
In 2019, more than 5,000 cases in China adopted using an ECMO, the report noted. In addition, the life-saving machine played a key role in curing patients with severe COVID-19 symptoms in Wuhan, Central China's Hubei Province.
For many years, it has been hard for China to break free from its dependence on the imports of such machines, as some core materials and technologies have not yet been mastered by Chinese companies, experts said.
According to Chinese Society of ExtraCorporeal Life Support, there were about 400 ECMO machines in China as of the end of 2018, with German's Maquet and Italy's Sorin accounting for the majority of the market in China.
"There is still a long way to go to produce ECMO machines domestically, but I think it is only a matter of time. For instance, on March 5, the medical equipment department of South China's Guangdong Province held meetings and discussed providing technical support to ECMO enterprises, in a bid to push forward the ECMO domestic-manufacturing process," Xu said.

