SOURCE / INDUSTRIES
Chinese partners may begin making Russia's Sputnik V vaccine in November
Chinese firms may begin making Russia’s vaccine
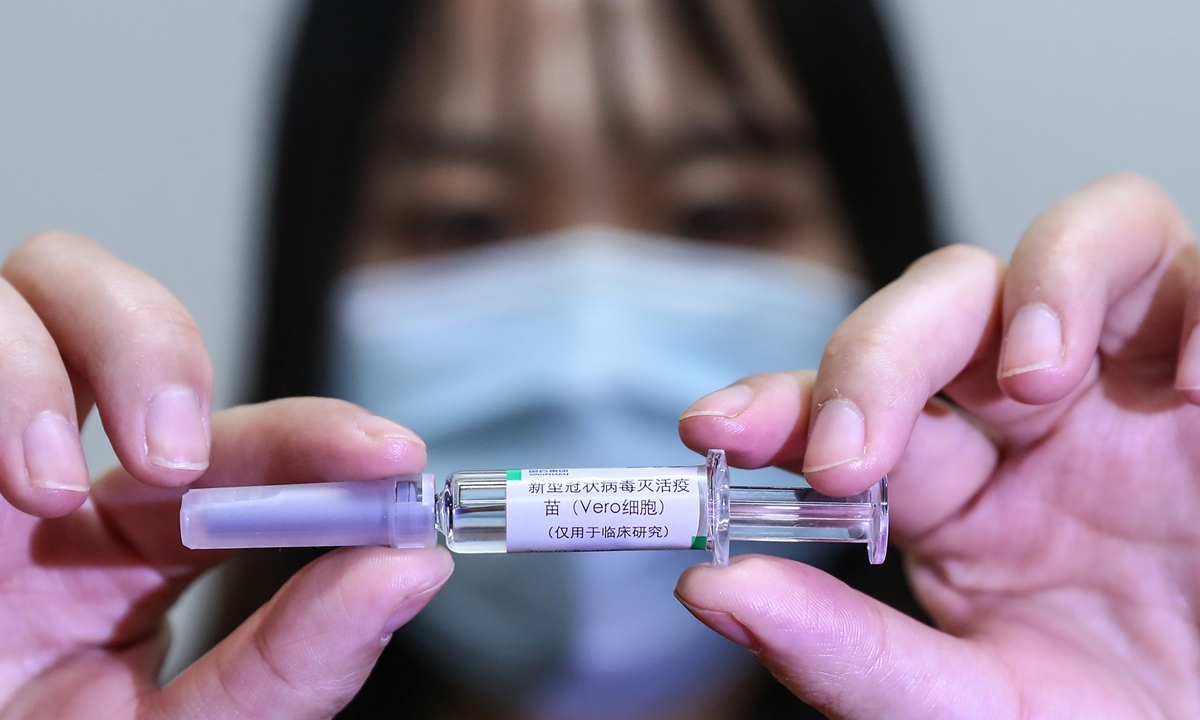
A medical worker shows the inactivated COVID-19 vaccine candidate developed by SinoPharm at the company's vaccine candidate production plant on April 10. Photo: Xinhua
The Russian Direct Investment Fund (RDIF), which provided funding for the Sputnik V vaccine, is in in talks with Chinese partners, producers and distributors and eyeing for production of the Russian vaccine as earlier as in November, the head of the Russian sovereign fund told the Global Times in an exclusive interview.
"Mass production in China and other countries could start in partnership with local partners. China has the ability to produce the vaccine and supply it - not only locally, but also by exporting it to other countries in Asia," said Kirill Dmitriev, CEO of the RDIF, noting that there is considerable interest in the Russian vaccine developed by the Gamaleya Center.
In early August, Russia surprised the world by unveiling its coronavirus vaccine and claiming a breakthrough in the global race on coronavirus research, although there have been some concerns over safety and efficacy as the vaccine has yet to complete its final clinical trials.
Dmitriev said the first batches of the vaccine have already been produced and full-scale production is expected to begin in September.
"Our Chinese partners may complete the technological transfer and start producing the Sputnik V vaccine as soon as November," Dmitriev said, without specifying which Chinese companies are involved.
Under pressure from the virus' health and economic onslaughts, countries around the world are betting heavily on vaccine research and development, with labs in China, the US, Europe and elsewhere looking for a potential vaccine.
Dmitriev said Russia has received preliminary applications for more than 1 billion doses of the vaccine from 20 countries and regions and is prepared to manufacture more than 500 million doses of the vaccine per year in five countries. Countries in Latin America, the Middle East, Asia and Africa have displayed the greatest interest in the vaccine, with a number of key contracts already finalized.
"The plan is to ramp up production capacity even higher," Dmitriev told the Global Times.
Dmitriev and his family have received the Sputnik V vaccine, and he said RDIF bet on the Gamaleya vaccine out of nearly three dozen candidates because it is based on the safest technology. The mechanism for introducing the genetic code of the virus spike into the human body is the safest, with studies into human adenoviruses as a potential base for a vaccine having begun as early as 1953.
Dmitriev also said the Gamaleya Center is using unique two-vector technology, which employs two different human adenoviral vectors (Ad5 and Ad26) for the first and second intakes of the vaccine to boost an immune response from the body, which means better efficacy.
Adenoviruses are also being used by scientists at the pharmaceutical giant Johnson & Johnson, the Chinese company CanSino Biologics and the University of Oxford in vaccine candidates.
"China's CanSino is using one of these vectors to develop its own vaccine. It is a good approach, but Russian scientists found that the immune response induced by this vaccine is weaker than the response to Sputnik V - because the second vector 'tricks the body', which develops some immunity to one type of vector after the first intake," said Dmitriev.
CanSino did not comment on the matter as of press time Monday.
"The Gamaleya two-different-vector approach could reduce irrelevant immune responses... Theoretically, it could be a better approach but trials and observations are needed before we can determine if this is the case," said a Beijing-based immunological expert, who asked not to be identified.
The expert told the Global Times that the potential Chinese vaccine-manufacturing partner could be no other company than CanSino, the sole Chinese company with successful experience in developing adenoviral vectors-based vaccines for Ebola.
"But the possibility of other Chinese companies cooperating with the Sputnik V project, such as Fosun Pharmaceutical, cannot be ruled out," the expert said.
Wang Ying, a Shanghai-based immunologist, said the two different human adenoviral vectors (Ad5 and Ad26) appraoch could mean better efficacy and relative safe. "The Sputnik V vaccine is worth to be anticipated."
Chinese authorities on August 16 granted the first invention patent to a domestically developed COVID-19 vaccine candidate, a recombinant adenovirus vaccine called Ad5-nCoV co-developed by CanSino Biologics and a team led by Chinese military infectious disease expert Chen Wei.
Separately, CanSino is reportedly cooperating with Russia's Petrovax to conduct a late-stage trial.