Real-world study of Pfizer's vaccine in Israel shows promising results against COVID-19 but requires long-term observation: expert
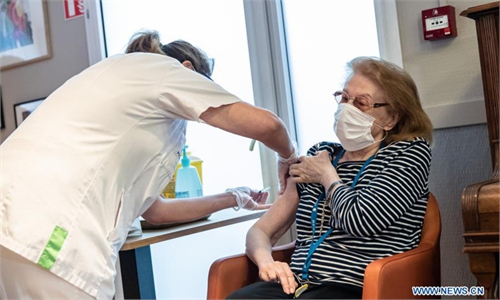
Photo: AFP
The first real-world study of vaccine efficacy, conducted in Israel, has shown that those who were inoculated with BioNTech/Pfizer's COVID-19 vaccine were 94 percent less likely to become ill.
So far, the efficacy data of COVID-19 vaccines under development and trial in the world are obtained under controlled conditions in clinical trials, but there is uncertainty about the vaccine's actual efficacy in real-world conditions.
However, a report based on data from Clalit Health Services (CHS), the largest of four integrated health care organizations in Israel, published on Wednesday by The New England Journal of Medicine, says that BioNTech/Pfizer's COVID-19 vaccine reduced symptomatic cases by 94 percent a week after the second dose, and prevented severe disease by 92 percent.
Israel has the world's fastest coronavirus vaccine program.
The data used in the report were collected from 1,163,534 vaccine recipients in Israel, from December 20, 2020, to February 1, 2021, according to the report. Its results are very similar to the Phase III clinical trials on the vaccine released last year.
Early data analysed by Public Health England (PHE) also showed the efficacy of BioNTech/Pfizer's COVID-19 vaccine is more than 85 percent after two shots, Reuters reported on Monday.
"Overall, we're seeing a really strong effect to reducing any infection, asymptomatic and symptomatic," PHE's strategic response director, Susan Hopkins, announced during a media briefing.
The data showed that the BioNTech/Pfizer's vaccine is highly effective in both preventing symptomatic and asymptomatic COVID-19 cases, while its efficacy on preventing symptomatic cases may be higher, Chinese experts said.
Zhuang Shilihe, a Guangzhou-based vaccine expert, told the Global Times on Thursday that research on vaccine efficacy in real-world conditions is more difficult, but more meaningful. Phase III clinical trials are conducted under controlled conditions, while the real world is full of unpredictable factors.
"It is very encouraging that the data from the real-world study on BioNTech/Pfizer's vaccine efficacy are highly consistent with the data from Phase III clinical trials, which confirms the vaccine's efficacy," Zhuang said.
Experts noted that more research is needed on the efficacy of a single shot of BioNTech/Pfizer's vaccine. The study in Israel showed that the protection against symptomatic COVID-19 illness for one shot of the vaccine is 57 percent; while PHE's data from the elderly group showed that a single shot is 57 percent effective against symptomatic COVID-19 disease.
Zhuang explained that one or two shots have very different meanings in terms of infectious disease prevention. The procedure to administer one shot is simple and facilitates the inoculation of a large population in a short period of time.
If the efficacy of one shot of the vaccine is comparatively high, when there is a world shortage of vaccine supply, people could be inoculated with only one shot to allow for an extension of the inoculation interval and extend coverage to as many people as possible, Zhuang said, noting that such methods must be supported by data.
The study on the data collected in Israel and the UK is still under scrutiny as the number of daily new cases in Israel have not decreased so far.