CORONAVIRUS
BioNTech to evaluate mixing vaccines as China enters final phase in approving mRNA shots: CEO
The COVID19 vaccine BNT162b2 co-developed by Fosun Pharma and BioNTech Photo: Courtesy of BioNTech
China's potential approval of BioNTech's mRNA COVID-19 vaccine has entered the final stage in decision-making processes, and the conversation with Chinese authorities is very "encouraging," German-based vaccine developer BioNTech's CEO Ugur Şahin told the Global Times in an exclusive interview on Thursday, with strong confidence for China's marketing prospects of the mRNA vaccine co-developed with Shanghai-based Fosun Pharma.
The developer is considering to evaluate the combination of mRNA-based vaccines with inactivated vaccines and adenovirus vector shots as a potential booster shots to stabilize the antibody level against the coronavirus once the immune response of antibodies wanes, Sahin said.
Sahin delivers his optimism about the potential official approval of its vaccine soon based on conversations with Chinese authorities.
Co-developers are working to submit all data sets required as soon as possible to support the decision of the authorities.
The vaccine is expected to be rolled out very quickly once it receives authorization, as relevant preparations including cold-chain delivery drills are in progress.
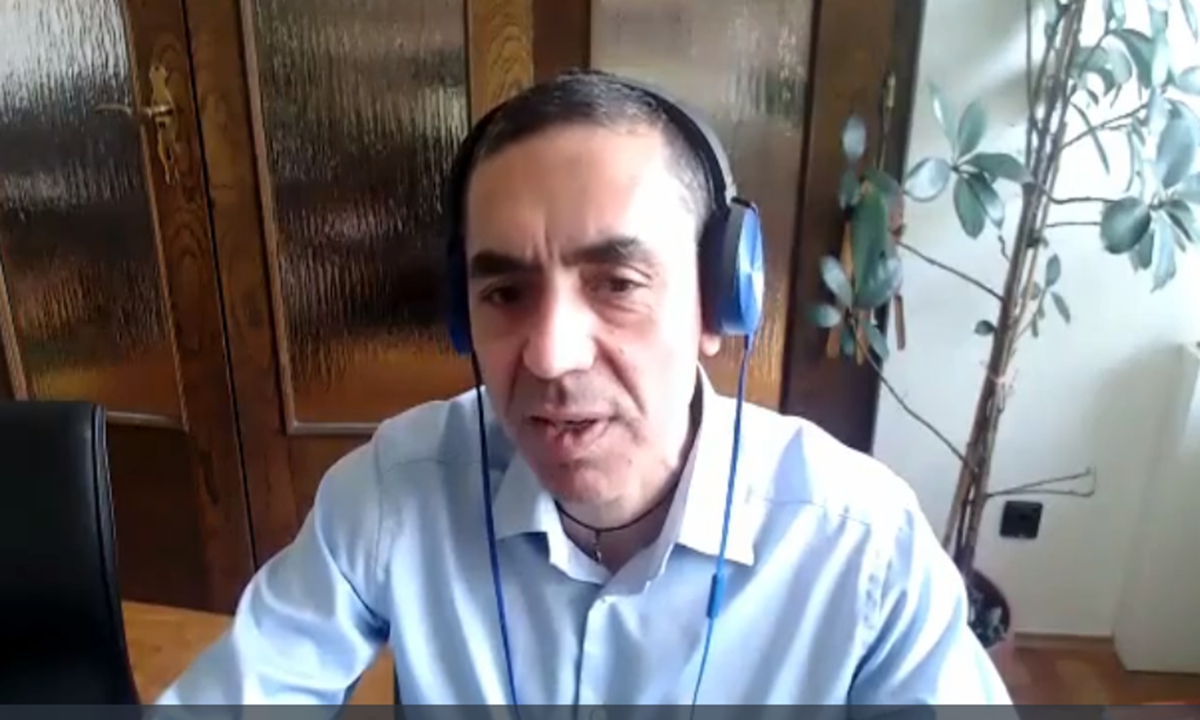
BioNTech CEO Ugur Sahin answers questions from the Global Times on Thursday. Screenshot from virtual interview
BioNTech provides the clinical supply of the vaccine from its GMP-certified mRNA manufacturing facilities, while Fosun specializes in clinical trial design and execution, regulatory approval and commercializing the vaccine in China.
The Shanghai government has held two virtual conferences with BioNTech's senior representatives since October, a positive sign for the vaccine's landing in the city that hosts many foreigners who want a BioNTech's shot for vaccine recognition when returning to their homeland.
BioNTech has gotten in touch with Chinese authorities since its collaboration with Fosun Pharma last year, and there have been multiple meetings among them afterward, Sahin noted.
Stressing that the booster shot will be helpful for longer immunity, Sahin said that "the antibody response after vaccination declines over time, which is normal for any type of vaccine."
"We have collected 6-month efficacy data from global trials, and found out we have 91 percent of efficacy, which is good. But what we have also seen is that we have a decline of antibodies," he said.
Based on the latest data, the vaccine can be stored at from 2 to 8C for a longer time, which provides better accessibility than its original requirement of ultra-low temperatures.
The developer is also making efforts to improve its formulation in a bid to extend the stability to last three months at 2 to 8 degrees, Sahin told the Global Times, adding that the new formulation is expected to be available in Autumn.
Out of concerns over coronavirus strains around the world, Sahin said that a research team has tested the vaccine's efficacy against more than 30 coronavirus variants, and it is "able to neutralize them in almost the same efficient manner," though with slight weakness against certain mutations.
If needed, the vaccine can be redesigned and improved within three months after approval.
The vaccine candidate is expected to be the first foreign-developed COVID-19 vaccine that China will import to pave the way for mixed shots in sequential inoculation, considering its good efficacy, Shao Yiming, China's leading physician-scientist and immunologist serving at the Chinese Center for Disease Control and Prevention, told the Global Times in a previous interview.