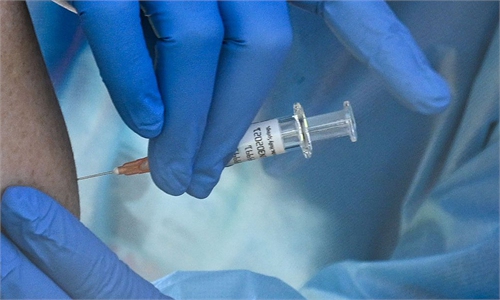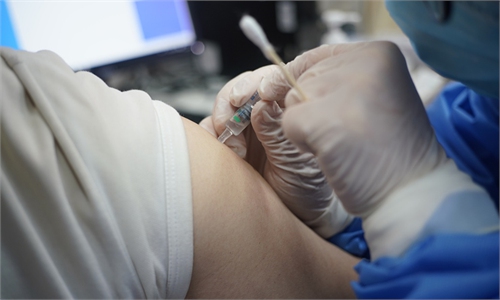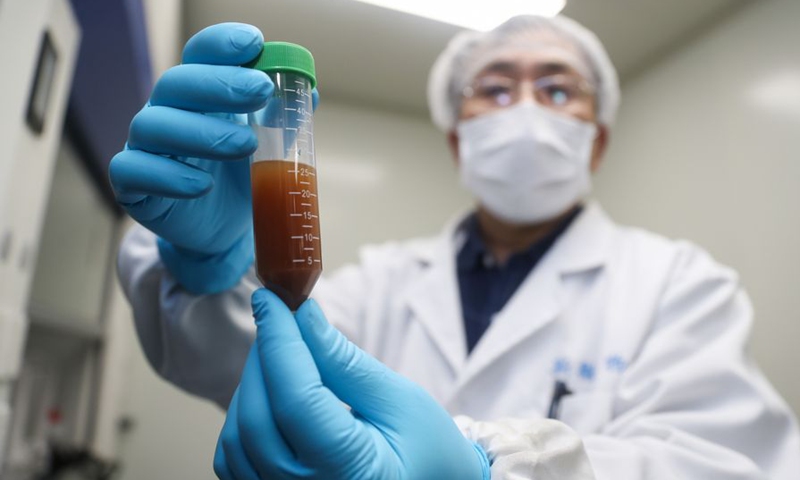
A researcher shows the experiment to develop an mRNA vaccine targeting the novel coronavirus in east China's Shanghai. (Photo: Xinhua)
A new Chinese mRNA COVID-19 vaccine, developed by scientists and medical personnel in Shanghai, started Phase I clinical trials among adults on Thursday.The vaccine was jointly developed by Shanghai East Hospital and Shanghai-based biotechnology company Stemirna Therapeutics, which also cooperated with the Chinese Center for Disease Control and Prevention (CDC) in the R&D process that began in January 2020, Shanghai East Hospital told the Global Times on Thursday.
The double-blind, placebo-controlled clinical trials of the vaccine will be conducted among COVID-19 susceptible people aged 19 and above, the hospital said.
Early in February 2020, samples of the vaccine were reportedly tested on more than 100 mice. It was approved by the Chinese government for clinical trials on January 4.
China is stepping up efforts in developing and producing mRNA COVID-19 vaccines. In December 2020, the construction of China's first mRNA COVID-19 vaccine production plant started in Yuxi, Southwest China's Yunnan Province. The plant is expected to produce 120 million doses of the vaccine per year, and construction is set to be completed within eight months.
Global Times