IN-DEPTH / IN-DEPTH
Progress made by Chinese vaccine developers shows protection of right to life a top priority of CPC
Editor's Note:
Who is the Communist Party of China (CPC)? What is the CPC's role in the new era?
The CPC has grown into one of the largest parties in the world in the process of leading the Chinese people in seeking liberation and happiness, making China as strong and prosperous as it is today.
As the CPC ushers the nation into a new era of development, the last decade has witnessed great achievements in national strength and prosperity, with people's confidence and recognition of this path rising to unprecedentedly high levels.
With more than 96 million members, the CPC concluded its 20th National Congress in October, which realized the goals of unifying thinking, fortifying confidence, charting the course for a new era, and boosting morale. The Global Times is publishing a series of stories to help the world understand the CPC in this new era, through stories from CPC members working on the frontlines of various fields, as well as through observations made by respected scholars.
In this installment, a Chinese vaccine expert shares his views on vaccine development over the last decade, along with the benefits reaped and challenges faced in the process.

When determining to work with a team in the development of a vaccine against the coronavirus disease on January 28, 2020, the vaccine expert Meng Weining did not expect that the speedy approach used to fight against COVID-19 could change the future of China's vaccinology.
The shortest time any vaccine had previously been developed, from viral sampling to approval, was four years, for mumps in the 1960s, according to the journal Nature. However, by January 2021, only one year later, the developers in Meng's team - a major domestic vaccine manufacturer Sinovac - had released results of all three phases of trials, and the fully-tested shot was put on the approval list of Indonesia for emergency use, while getting a nod of approval from the Chinese health authority for emergency use in February 2021.
"Pride, but at high stakes" is a phrase Meng, chairman of the quality management committee of Sinovac, used to say when answering questions about his feelings regarding his work in virology and vaccinology in his over 15-year-long career. In Meng's eyes, the last decade was one that's seen obvious progress albeit through various challenges.
As of June 2022, China has provided more than 2.2 billion doses of the COVID-19 vaccine to more than 120 countries and international organizations, transferred technology to and cooperated in vaccine production with more than 20 countries, and formed an annual production capacity of one billion doses of the COVID-19 vaccine overseas.
Behind this boost in both quality and international confidence are the unremitting efforts of the Chinese government, virologists, and state-of-the-art academic labs which accelerated their efforts amid risks of rapid viral mutations, questions about safety, and the challenges of producing enough doses for billions of people.
Industrial observers have noted that the rapid development of China's vaccine industry over the last decade couldn't have been achieved without the CPC placing the Chinese people's right to life as a top priority and its strong support for biomedicine.

COVID-19 vaccines R&D
Compressing a process that can take a decade into roughly 10 months without sacrificing vaccine safety is what Meng calls the journey of developing the COVID-19 vaccine miracle.
But over the course of the year, Meng has suffered from bouts of anxiety and insomnia.
At the beginning, like many global enterprises, Meng's team was dogged by uncertainties about whether their COVID-19 vaccine would prove effective, and whether a rush to the finish line would compromise safety. To help mitigate such concerns, the team opted for a more mature technology route.
"I still remember what a heart-stopping moment we went through when we went to Brazil in November 2020 at the height of the pandemic to do the clinical trial, which was our unprecedented large-scale clinical trial overseas," Meng told the Global Times. "We surprisingly heard of one death that occurred among our trial participants, and the Brazilian health authority immediately suspended the trial amid media and public outcry concerning the potential uncontrolled side effects of the vaccine."
The next few hours marked the most nerve-wracking period in Meng's career, because it could have meant that the entire team's hard work and effort would be naught.
Fortunately, a few hours later the investigation revealed that the "severe adverse event" was not related to the vaccine but likely a suicide. The experiment was then immediately resumed.
"We would work in the lab in the day, and turn on the news channels in the night to see that the top news were all about the thing that we were working hard for, and that was amazing," said Meng.
With the global attention, the Chinese COVID-19 vaccines became one of the first batches of vaccines that was launched and entered the international market. Meng called it a landmark moment in China's vaccinology history and a major breakthrough for the Chinese drug regulatory authorities.
What made the development and testing processes so fast? Meng underlined the lessons learned from China's huge market and strong full-chain vaccine production system.
On the one hand, China was highly efficient in virus detection as it was able to decode the genetic sequence of the coronavirus disease in a matter of few days, much shorter than the previous detection of the SARS virus sequence in 2003 which took about a month. On the other hand, China's self-sufficiency in producing all components and facilities used on the vaccine production chain also accelerated the rollout, said Meng.
The efficient operation mechanisms inherent in a socialist society boosted this process. The CPC, with people's interests as a core motivator, immediately decided to invest in vaccine R&D at the very beginning, setting up a special team to coordinate and guide vaccine research, and provided important support to manufacturers in the coordination of resources in various links, Meng noted.
Institutional guarantee
According to a report on China's vaccine industry between 2018 and 2022, China has become the world's largest vaccine producer and consumer, with more than 40 vaccine manufacturers supplying more than 1 billion doses to the market annually.
China is also one of the few countries in the world that can independently produce vaccines against viruses targeted by state-funded immunization programs, and central government's spending on these efforts has exceeded $4 billion a year, official data shows. Currently, China is at an all-time low for many vaccine-preventable infectious diseases.
In 2018, the CPC sacked multiple provincial and local officials and vowed to punish a pharmaceutical company over a vaccine scandal that ignited public anger over the safety of domestically produced pharmaceuticals. The move aimed to shore up public confidence in the pharmaceutical industry after a primary rabies vaccine producer was revealed to have fabricated data, with the company's product recalled, ceasing further production.
Later, in December 2019, China's new vaccine administration law went into effect. According to the law, the supervision of vaccines will cover the whole process from vaccine development, production, and distribution, to vaccination. The law also imposes tough punishments on wrongdoers, stipulating that people whose violations constitute a crime shall bear heavier criminal responsibility, the Xinhua News Agency reported.
Vaccine scandals over the last decade have indeed shaken public confidence in domestic vaccines. But the CPC's swift response shows its determination to salvage the vaccine industry's credibility, said Meng. "This also reflects the CPC's stricter supervision over the biomedical industry and its emphasis on people's right to life and health."
The WHO on August 23 ranked China's vaccine regulatory system at a functional level of maturity according to the WHO's global classification system for national medical product regulatory authorities, which experts said means that China has a stable, well-functioning, and integrated regulatory system to ensure the quality, safety, and efficacy of vaccines that are manufactured, imported, or distributed in the country, and lays an important foundation for Chinese vaccines to be imported by other countries.
Meng remains impressed by an incident during clinical trials in Chile when the owner of the hotel where he was staying presented Meng and his colleagues with special local products - wine and small gifts - along with a note: "Thank you so much for the efforts you've put in for us."
Who is the Communist Party of China (CPC)? What is the CPC's role in the new era?
The CPC has grown into one of the largest parties in the world in the process of leading the Chinese people in seeking liberation and happiness, making China as strong and prosperous as it is today.
As the CPC ushers the nation into a new era of development, the last decade has witnessed great achievements in national strength and prosperity, with people's confidence and recognition of this path rising to unprecedentedly high levels.
With more than 96 million members, the CPC concluded its 20th National Congress in October, which realized the goals of unifying thinking, fortifying confidence, charting the course for a new era, and boosting morale. The Global Times is publishing a series of stories to help the world understand the CPC in this new era, through stories from CPC members working on the frontlines of various fields, as well as through observations made by respected scholars.
In this installment, a Chinese vaccine expert shares his views on vaccine development over the last decade, along with the benefits reaped and challenges faced in the process.

A research and development team shows Sinovac inactivated COVID-19 vaccine. Photo: Courtesy of Sinovac
When determining to work with a team in the development of a vaccine against the coronavirus disease on January 28, 2020, the vaccine expert Meng Weining did not expect that the speedy approach used to fight against COVID-19 could change the future of China's vaccinology.
The shortest time any vaccine had previously been developed, from viral sampling to approval, was four years, for mumps in the 1960s, according to the journal Nature. However, by January 2021, only one year later, the developers in Meng's team - a major domestic vaccine manufacturer Sinovac - had released results of all three phases of trials, and the fully-tested shot was put on the approval list of Indonesia for emergency use, while getting a nod of approval from the Chinese health authority for emergency use in February 2021.
"Pride, but at high stakes" is a phrase Meng, chairman of the quality management committee of Sinovac, used to say when answering questions about his feelings regarding his work in virology and vaccinology in his over 15-year-long career. In Meng's eyes, the last decade was one that's seen obvious progress albeit through various challenges.
As of June 2022, China has provided more than 2.2 billion doses of the COVID-19 vaccine to more than 120 countries and international organizations, transferred technology to and cooperated in vaccine production with more than 20 countries, and formed an annual production capacity of one billion doses of the COVID-19 vaccine overseas.
Behind this boost in both quality and international confidence are the unremitting efforts of the Chinese government, virologists, and state-of-the-art academic labs which accelerated their efforts amid risks of rapid viral mutations, questions about safety, and the challenges of producing enough doses for billions of people.
Industrial observers have noted that the rapid development of China's vaccine industry over the last decade couldn't have been achieved without the CPC placing the Chinese people's right to life as a top priority and its strong support for biomedicine.

Meng Weining
COVID-19 vaccines R&D
Compressing a process that can take a decade into roughly 10 months without sacrificing vaccine safety is what Meng calls the journey of developing the COVID-19 vaccine miracle.
But over the course of the year, Meng has suffered from bouts of anxiety and insomnia.
At the beginning, like many global enterprises, Meng's team was dogged by uncertainties about whether their COVID-19 vaccine would prove effective, and whether a rush to the finish line would compromise safety. To help mitigate such concerns, the team opted for a more mature technology route.
"I still remember what a heart-stopping moment we went through when we went to Brazil in November 2020 at the height of the pandemic to do the clinical trial, which was our unprecedented large-scale clinical trial overseas," Meng told the Global Times. "We surprisingly heard of one death that occurred among our trial participants, and the Brazilian health authority immediately suspended the trial amid media and public outcry concerning the potential uncontrolled side effects of the vaccine."
The next few hours marked the most nerve-wracking period in Meng's career, because it could have meant that the entire team's hard work and effort would be naught.
Fortunately, a few hours later the investigation revealed that the "severe adverse event" was not related to the vaccine but likely a suicide. The experiment was then immediately resumed.
"We would work in the lab in the day, and turn on the news channels in the night to see that the top news were all about the thing that we were working hard for, and that was amazing," said Meng.
With the global attention, the Chinese COVID-19 vaccines became one of the first batches of vaccines that was launched and entered the international market. Meng called it a landmark moment in China's vaccinology history and a major breakthrough for the Chinese drug regulatory authorities.
What made the development and testing processes so fast? Meng underlined the lessons learned from China's huge market and strong full-chain vaccine production system.
On the one hand, China was highly efficient in virus detection as it was able to decode the genetic sequence of the coronavirus disease in a matter of few days, much shorter than the previous detection of the SARS virus sequence in 2003 which took about a month. On the other hand, China's self-sufficiency in producing all components and facilities used on the vaccine production chain also accelerated the rollout, said Meng.
The efficient operation mechanisms inherent in a socialist society boosted this process. The CPC, with people's interests as a core motivator, immediately decided to invest in vaccine R&D at the very beginning, setting up a special team to coordinate and guide vaccine research, and provided important support to manufacturers in the coordination of resources in various links, Meng noted.

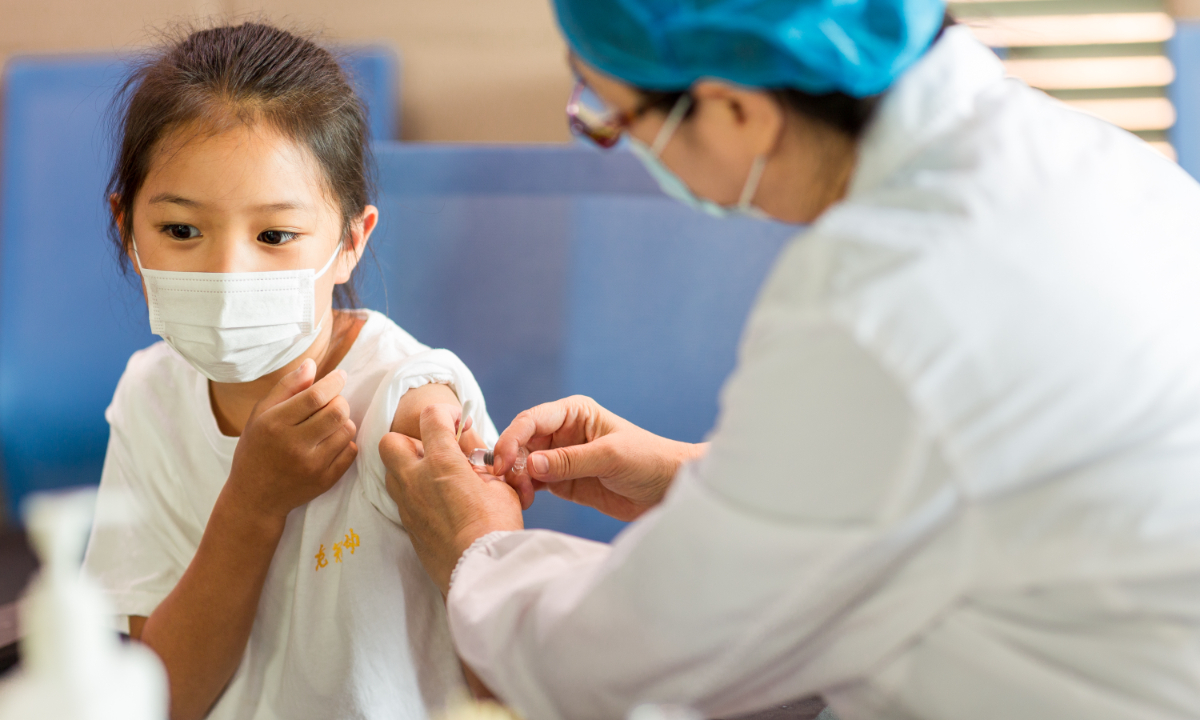
A young girl receives an inactivated COVID-19 vaccine. Photo: Courtesy of Sinovac
Institutional guarantee
According to a report on China's vaccine industry between 2018 and 2022, China has become the world's largest vaccine producer and consumer, with more than 40 vaccine manufacturers supplying more than 1 billion doses to the market annually.
China is also one of the few countries in the world that can independently produce vaccines against viruses targeted by state-funded immunization programs, and central government's spending on these efforts has exceeded $4 billion a year, official data shows. Currently, China is at an all-time low for many vaccine-preventable infectious diseases.
In 2018, the CPC sacked multiple provincial and local officials and vowed to punish a pharmaceutical company over a vaccine scandal that ignited public anger over the safety of domestically produced pharmaceuticals. The move aimed to shore up public confidence in the pharmaceutical industry after a primary rabies vaccine producer was revealed to have fabricated data, with the company's product recalled, ceasing further production.
Later, in December 2019, China's new vaccine administration law went into effect. According to the law, the supervision of vaccines will cover the whole process from vaccine development, production, and distribution, to vaccination. The law also imposes tough punishments on wrongdoers, stipulating that people whose violations constitute a crime shall bear heavier criminal responsibility, the Xinhua News Agency reported.
Vaccine scandals over the last decade have indeed shaken public confidence in domestic vaccines. But the CPC's swift response shows its determination to salvage the vaccine industry's credibility, said Meng. "This also reflects the CPC's stricter supervision over the biomedical industry and its emphasis on people's right to life and health."
The WHO on August 23 ranked China's vaccine regulatory system at a functional level of maturity according to the WHO's global classification system for national medical product regulatory authorities, which experts said means that China has a stable, well-functioning, and integrated regulatory system to ensure the quality, safety, and efficacy of vaccines that are manufactured, imported, or distributed in the country, and lays an important foundation for Chinese vaccines to be imported by other countries.
Meng remains impressed by an incident during clinical trials in Chile when the owner of the hotel where he was staying presented Meng and his colleagues with special local products - wine and small gifts - along with a note: "Thank you so much for the efforts you've put in for us."
The COVID-19 vaccine has proven that China is capable of supplying safe and effective vaccines. This is a good opportunity for those who have doubts about Chinese vaccines to review their biases, Meng suggested.
"As vaccine developer, we appreciate how the WHO expected Chinese vaccine makers to get more involved in the global market, alongside Indian producers, to counterbalance Western vaccine oligopolists. The WHO's trust in China's vaccine quality and regulatory standards has increasingly deepened. This is a hard-won result," Meng said.
"As vaccine developer, we appreciate how the WHO expected Chinese vaccine makers to get more involved in the global market, alongside Indian producers, to counterbalance Western vaccine oligopolists. The WHO's trust in China's vaccine quality and regulatory standards has increasingly deepened. This is a hard-won result," Meng said.