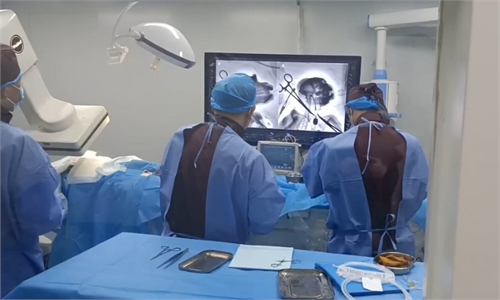
A BCI product, Xhand, is showcased at China (Shanghai) International Technology Fair on April 15, 2021. Photo: VCG
Reacting to news of Neuralink's brain-computer interface (BCI) chip implantation, Chinese scientists on Tuesday pointed to China's rapid progress in BCI research, while emphasizing the need for synergy between research and industry application.
In a notable advance, American entrepreneur Elon Musk announced on Tuesday that his start-up Neuralink Corp had implanted a BCI chip in a human patient. Writing on X, Musk said that the patient is "recovering well," with promising initial test results, stirring global interest in this burgeoning field.
Chinese experts are optimistic about the advances in the BCI sector. On Monday, a collaborative effort between Tsinghua University and Xuanwu Hospital Capital Medical University led to a breakthrough in BCI rehabilitation using neural electronic opportunity technology. The patient demonstrated more than 90 percent accuracy in using a pneumatic glove for various tasks, showcasing China's capabilities in this domain.
However, Zhang Qiang, a professor at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, said that while China has made significant strides in deep brain stimulation (DBS) research, its application in human BCI trials is still evolving.
China has witnessed rapid development in BCI research. For instance, Li Luming, a professor at Tsinghua University and his team, pushed the commercial use of DBS technology. Zhejiang University conducted human experiments with invasive BCIs, and Nankai University has been active in interventional BCI research, Duan Feng, a professor at the College of Artificial Intelligence, Nankai University, told the Global Times on Tuesday.
But there are challenges, particularly in commercialization and funding of BCI research. The experiments are expensive and require substantial funding to move from research to commercialization, Duan said.
On May 4, 2023, a team led by Duan collaborated with the Chinese People's Liberation Army General Hospital and Shanghai HeartCare Medical Technology Co, as they conducted the first interventional BCI trial on a non-human primate.
While Neuralink's success in human implant trials sets a high benchmark, Chinese researchers are optimistic that China can reach similar standards in sensitivity and biocompatibility, although a gap still exists in moving toward human clinical trials.
Zhang's team has been at the forefront of BCI research, having developed a new topological hydrogel, but it has limited funding.
As a field at the forefront of scientific exploration, the uncertain commercial potential of BCI research means there are few enterprises willing to invest.
In a recent development, the Ministry of Industry and Information Technology, along with six other ministries, recognized the BCI industry as a key area of innovation in China's plans for industries of the future.
The inclusion underscores the anticipated role of BCIs in China's health and technology sectors and marks a significant step in addressing the challenges faced by researchers in this advanced field.