China leads vaccine R&D
By Leng Shumei, Hu Yuwei and Chu Daye Source:Global Times Published: 2020/4/15 0:48:40
2 million confirmed global COVID-19 cases, joint platform urged

A staff member displays a sample of the COVID-19 inactivated vaccine at a vaccine production plant of China National Pharmaceutical Group (Sinopharm) in Beijing, capital of China, April 10, 2020. Photo:Xinhua
As the number of coronavirus infection cases rapidly approaches two million worldwide and as the world grows more desperate by the day for a vaccine that could put an end to the COVID-19 pandemic, a glimpse of hope finally emerged from China: not one but three domestically developed vaccines have entered clinical trials. However, even as Chinese scientists and researchers are working around the clock to push forward vaccines, global cooperation remains paramount to not only accelerating research and development (R&D) of vaccines but also paving the way for a more collaborative platform for future crises.
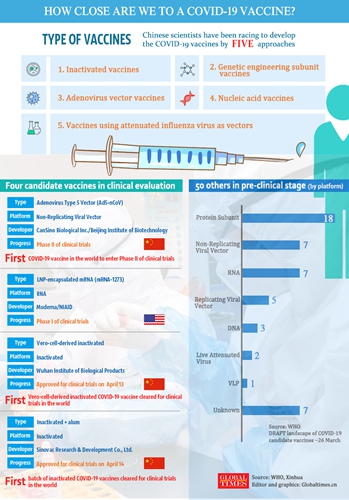
Two inactivated vaccines for the coronavirus developed in China have got the green light from authorities to enter into clinical trials to have phase I tests following one developed by a research team led by a top Chinese military epidemiologist which has entered Phase II trials last week, Wu Yuanbin, director-general of science and technology for social development with the Ministry of Science and Technology, said at a press conference on Tuesday.
The adenovirus vector vaccine, which was developed by the team led by military infectious disease expert Chen Wei, has entered into Phase II clinical tests and as of 5 pm Monday, 273 volunteers received the vaccine including an 84-year-old man from Wuhan. The vaccine is the only one in the world to enter into phase II clinical tests.
The Phase I trial is aimed to evaluate the safety and immunization of the vaccine, while Phase II and III will assess its effectiveness.
There have been at least 70 coronavirus vaccines worldwide, with more than 60 R&D research teams in more than 10 countries getting involved, in a race to find a cure for the pathogen, including frontrunner pharmaceutical companies, biotech enterprises, government-supported professionals, and universities.
According to a WHO report, there are also two other vaccines in the world that are close to Phase II clinical trials as of Tuesday, which were separately developed by US drugmakers Moderna Inc. and Inovio Pharmaceuticals Inc.
China's vaccine research and development capacity have ranked among the top across the world. China especially leads the world in traditional productive technology and developing inactivated vaccines because it started research earlier on this method, according to experts.
Wu said on Tuesday's conference that Chinese experts are trying five different technical methods in developing the vaccines, including the inactivated one and nucleic acid vaccine. These vaccines are also expected to apply for clinical trials in April and May.
While a vaccine is widely viewed as a key weapon for people to overcome the COVID-19 pandemic and establish herd immunity against the coronavirus, experts noted that it still needs six to 12 months before a COVID-19 vaccine can be used for emergency usage despite the quick development and green channel offered by the authorities.
And it requires global cooperation on the research, which is full of uncertainty and challenges, they said.

Zhu Aobing takes his vaccine injection on March 19 in Wuhan. Photo: Courtesy of Zhu Aobing
A vaccine, if successful, will be put into use in China first before being promoted to the international market, which requires the support of international organizations such as WHO and the certification of medical administrations in other interested countries, a Beijing-based immunology expert, who asked to remain anonymous told the Global Times on Tuesday.
While it will be easier for countries with comparatively weak research abilities to accept China's vaccines, experts noted that there possibly would be difficulties for China's vaccine to enter European countries and the US.
As the epidemiology of COVID-19 might differ by geography, a vaccine developed in China is also required to take at least three phases of a human clinical trial to test its efficacy and adaption in other countries, which costs a certain time.
More crucially, the vaccine developed by the Chinese teams has to meet local evaluation standards if it is planned to be released on the market in other regions like the US or EU, which poses a challenge for vaccine exports and requires more transnational cooperation, the anonymous expert said.
But China's experiences in promoting the Ebola vaccine to Africa under the support of WHO may help, which has shown the world China's ability in producing qualified and effective vaccines, experts noted.
In 2014, China approved the recombinant Ebola vaccine developed by Chen Wei's team and tested in Sierra Leone, one of the areas most affected by the Ebola outbreak in 2015. The experience in Ebola vaccine development also contributes to COVID-19 vaccine development.
China has demonstrated to the world that it is an active and responsible player in the global battle against the pandemic but some countries are still treating it as a zero-sum game. Global cooperation to deal with the pandemic is urgently needed with experts calling for the establishment of an integrated mechanism under the idea of the community of shared future.
According to a statement WHO issued on Monday, a group of experts with diverse backgrounds are working toward the development of vaccines against COVID-19 under the organization's coordination.
The statement has attached a declaration signed by more than 100 experts worldwide including Qin Chuan, a professor from the Institute of Laboratory Animal Sciences, the Chinese Academy of Medical Sciences and Peking Union Medical College.
Xu Nanping, China's vice-minister of science and technology, said at a press conference on March 26 that China stresses global cooperation on vaccine development from the very beginning of the research: share the virus' gene sequence with the world and cooperate with foreign companies.
For example, Chinese companies are working with Inovio on DNA vaccines; with German-based BioNTech on mRNA vaccines and with UK-based drugmaker GlaxoSmithKline GSK on recombinant vaccines, according to Xu.
Reflections from the pandemic
The COVID-19 pandemic, which has infected nearly two million with about 120,000 deaths as of Tuesday, has made the world realize its vulnerability in the face of nature.
"Coronaviruses were discovered in the 1960s, but a vaccine on such virus was never developed despite intensive efforts from global researchers. This is mainly due to limited understanding of the complexity of the laws of coronavirus immunity and, secondly, to fewer people and less money being spent on the development of coronavirus vaccines," said the anonymous expert.
During the Severe Acute Respiratory Syndrome (SARS) outbreak in 2003, a group of world-leading researchers and institutions working on a coronavirus vaccine emerged in China, but then faded away and trials were halted because many international experts did not expect another coronavirus to strike again so quickly, the anonymous expert, who worked closely on the coronavirus vaccine against SARS, told the Global Times.
As the most superabundant biological creature on Earth, most viruses have developed a pathogenic relationship with their hosts, meaning they can cause diseases ranging from a mild cold to far more serious conditions, the expert said.
But experts try to ease the public not to be overly afraid of viruses, because the body's immune system allows us to resist most viruses, and many viruses in nature are not deadly to humans.
Meanwhile, as technology advances, humans are increasingly taking the initiative in the fight against viruses, experts suggested, adding that people's understanding of viruses has also improved greatly since the 1918 pandemic (H1N1 virus), and people are now able to understand virus' origins by analyzing their genetic sequences.
Learning from the tough COVID-19 pandemic, the Chinese central government in February has urged an improvement in the preventive mechanism in response to major public health crises and the country's public health emergency and management system, calling for a biosecurity law as soon as possible.
Experts said that strategic mechanism and law are the basis to ensure consistent research and preparation for future biosecurity crises but, more importantly, the public and policy performers need to expand the horizon and get prepared for a possible crisis in the future.
Hosted by the Johns Hopkins Center for Health Security, the US initiated a pandemic simulation exercise, named "Event 201," in October 2019 in the New York City to spotlight preparedness gap in facing biological threats, pandemics, and other disasters in social communities.

Staff members talk beside the virus culture/inactivation area of a vaccine production plant of China National Pharmaceutical Group (Sinopharm) in Beijing, capital of China, April 10, 2020. Photo:Xinhua
The simulations started with 2001's Dark Winter, a senior-level bio-terrorist attack simulation, which gathered US security officials for its simulated widespread smallpox attack. Such exercise is believed to be influential in reinforcing US disaster response systems.
Some places in China have also conducted exercises dealing with public safety emergencies. For example, Wuhan's authority launched an exercise for infectious disease prevention and control before the 7th International Military Sports Council (CISM) Military World Games held in Wuhan, Central China's Hubei Province in October 2019. Hubei is the region hit hardest in Chinaby the COVID-19 epidemic.
The US' simulations are a strategic move targeted at the future while the exercises in China are limited in specific events, more stressing operations, Wang said, noting that both sides are important for a complete emergency system.