Massive needs accelerate worldwide vaccines race
By Leng Shumei and Hu Yuwei Source: Global Times Published: 2020/11/25 23:05:19
China’s leading companies ready for large-scale sales
An illustration shows vials of a COVID-19 vaccine and syringes with the logos of US pharmaceutical company Pfizer and German partner BioNTech, released on Tuesday.Photo: AFP
November has witnessed an information explosion of global COVID-19 research and development. In the 16 days from November 9 to 25, three Western vaccine producers - Pfizer, Moderna and AstraZeneca - have revealed their interim Phase-III clinical trial data, and two Chinese companies - Sinovac and Sinopharm - separately unveiled plans on data publishing or applications for marketing.
China's leading vaccine producer China National Pharmaceutical Group (Sinopharm) on Wednesday announced that it has submitted an application to Chinese authorities for marketization of its COVID-19 vaccine.
This phenomenon indicates that the global vaccine race has turned white-hot as more and more countries are eager to roll out massive vaccination programs in face of the exhausting and deadly pandemic, observers said, adding that these countries also fear a sharp vaccine shortage once viable candidates are available.
At least five countries - Japan, the US, UAE, Russia and Spain - had announced free vaccination plans for the public as of Tuesday.

Inactivated COVID-19 vaccine CoronaVac produced by Chinese vaccine developer Sinovac Photo: Courtesy of Sinovac
Brazil, which is cooperating with China's Sinovac Biotech on Phase-III clinical trials for its vaccine candidate, is also in high gear to lay out an urgent vaccination plan.
According to a document the United Nations Children's Fund (UNICEF) released in August, the global demand for COVID-19 vaccines will reach 1.3 billion doses by the end of 2020, 1.8 billion by June 2021 and 7.4 billion by the end of 2021, 13.5 billion by the end of 2022 and 14.1 billion by the end of 2023.
Meanwhile, supply capacity will be 110 million doses by the end of 2020, 5.4 billion by the end of 2021 and 15.8 billion by the end of 2022. It will then stay at the production level, according to the UNCEF document, although this is seemingly far less than the actual production that can be expected, according to data released by global vaccine producers.
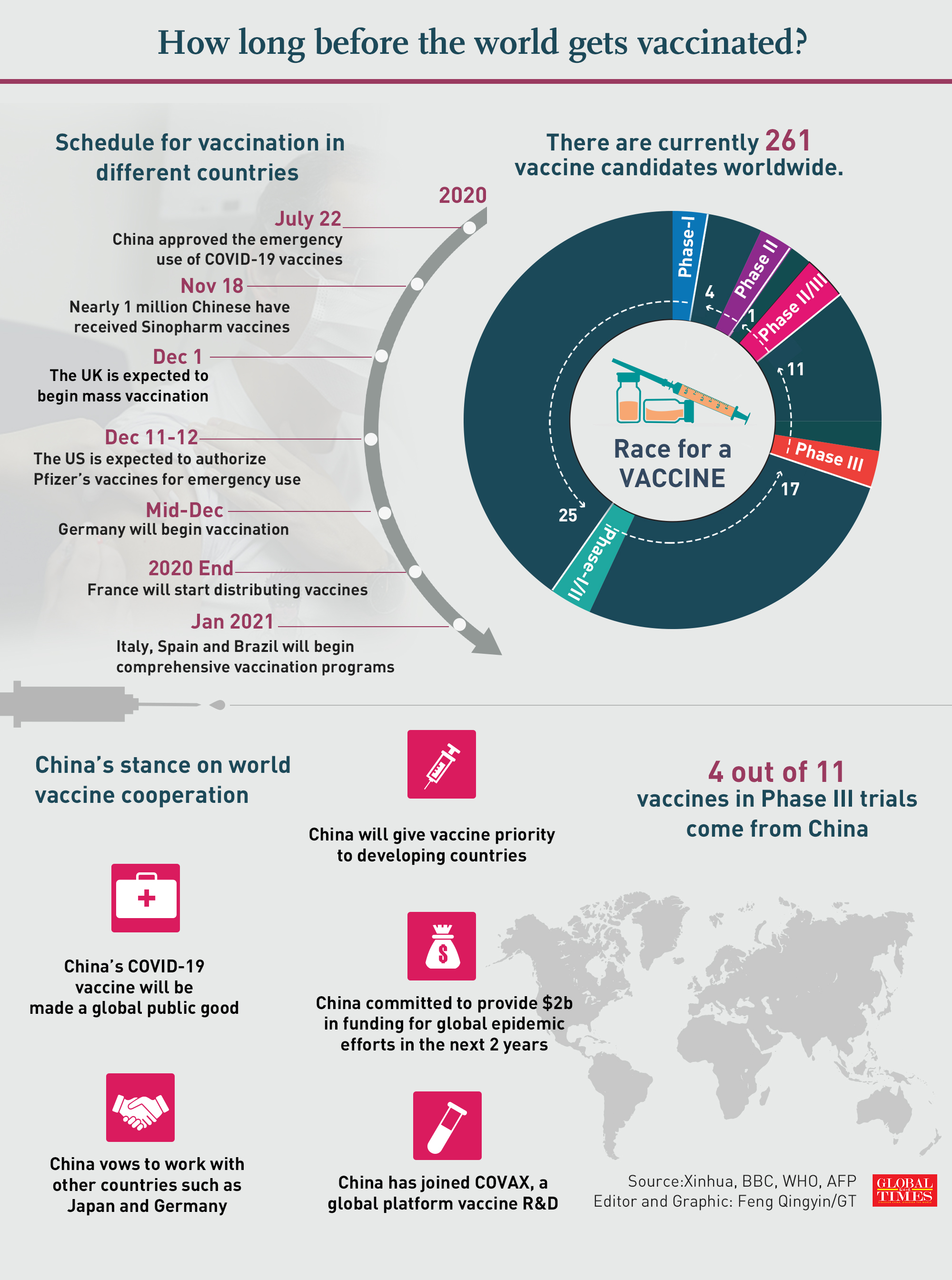
Infographic:GT
Promising prospects
China's national health officials announced in September that the country would be able to produce 6 million doses of COVID-19 vaccines by the end of 2020.
Sinopharm said that they would be able to produce 1 billion doses next year.
A representative from Sinopharm told the Global Times on Wednesday that Sinopharm had collected data from major countries carrying out clinical trials of its vaccine, such as the UAE. The results are expected to be good but it is up to Chinese authorities to make the decision as the authorities have strict review standards.
"Our data is evaluated by relevant departments following protocols even stricter than those in some Western countries, and we are in close communications with the WHO," said the representative who asked for anonymity.
It will be possible to release the Phase-III trial data in academic journals after approval is given, according to the representative.
Chinese pharmaceutical company Fosun Pharma, the Chinese partner of German vaccine producer BioNTech's vaccine BNT162b2, also told the Global Times that Phase-II clinical trials of the vaccine had kicked off on Tuesday in Taizhou and Lianshui in East China's Jiangsu Province.
The trials will cover 960 healthy adults aging from 18 to 85. The trial results will not only provide key data for the launch of the vaccine in China , but may also play a positive role in the widespread use of the vaccine throughout Asia and around the world, according to a statement Fosun Pharma sent to the Global Times on Wednesday.
Pfizer, which is the partner of BioNTech outside China, also reportedly plans to manufacture 50 million doses of the vaccine by the end of this year and increase its production to 1.3 billion doses next year.
Moderna said in September that it was on track to produce 20 million doses of its experimental coronavirus vaccine by the end of the year, while maintaining its goal of readying 500 million to 1 billion doses in 2021, Reuters reported.
Pfizer and Moderna, after releasing interim data on their candidates earlier this month, both said they would file applications with the US Food and Drug Administration for urgent use.
Media reported that Moderna has reached supply agreements with some countries and regions in North America and the Middle East.
AstraZeneca says it is making "rapid progress" in terms of manufacturing with a capacity to produce up to 3 billion doses of the vaccine next year, according to CNBC on Monday.
Russia also reportedly aims to produce 1 billion doses of its vaccine in 2021.
Health worker and volunteer Fabiana Souza receives a COVID-19 vaccine produced by Chinese company SinoVac Biotech at the Sao Lucas Hospital, in Porto Alegre, southern Brazil on August 8. Photo: AFP
International cooperation
Some Western media have compared the vaccine race during this year's pandemic with last century's "gold rush," which was full of favor of money and power. They described China's efficient vaccine research and development and kind offer of vaccines to countries and regions in need as revealing the country's ambition to use the vaccine as tool to play geopolitics. Chinese experts refuted this allegation as a distortion of China's sincerity to help promote global public health and a politicization of China's vaccines.
As the pandemic situation in China is essentially under control, Chinese central government and companies have been actively offering support to countries in need of vaccines, with Chinese leaders reiterating on many occasions that the country will provide its vaccines as a public good accessible and affordable to people around the world.
In a phone conversation with German Chancellor Angela Merkel on Tuesday, Chinese President Xi Jinping said that China stands ready to strengthen exchanges and cooperation with Germany on COVID-19 vaccines and push for the vaccines to be fairly distributed as a global public good, especially for the benefit of developing countries.
China and Germany have been cooperating on combating the pandemic, including the development of vaccines throughout the outbreak.
Fosun Pharma and BioNTech reached collaboration on the vaccine on March 13, 2020. The company is in charge of developing and commercializing COVID-19 vaccine products based on the BioNTech mRNA technical platform in the Chinese mainland as well as China's Hong Kong, Macao and Taiwan regions, read the statement.
Fosun Pharma has also been deeply engaged in the development of the vaccine, participating in the discussion over development plans, completing clinical trials in China and designing and completing animal challenge trails of BioNTech's selected mRNA vaccine candidates including BNT162b2, according to the statement.
The two sides' collaboration has progressed well under the support of the German and Chinese governments, a representative from Fosun Pharma told the Global Times.
China also agreed on Tuesday to enhance cooperation with Japan in fighting the epidemic during Chinese State Councilor and Foreign Minister Wang Yi's ongoing visit to Japan.
Japanese vaccine producer I'rom Group is reportedly cooperating with the Shanghai Public Health Clinic Center on a vaccine.
As to the supply, according to media reports, Chinese vaccine company CanSinoBIO in October agreed to provide 35 million doses of its experimental vaccine to Mexico. Sinovac Biotech LTD signed a contract on September 30 with the government of Brazil's Sao Paulo state to provide 46 million doses of its vaccine.
China's repeated promise to provide its vaccines as a public good indicates the country's responsibility when the world is facing such an unprecedented health disaster, experts noted.
Unlike Western countries and companies that have engaged too many capital and geopolitical factors in vaccines - for example, some of the Western companies may scramble to release clinical trial data to attract investment rather than to provide hope for people suffering from the novel coronavirus - China pays more attention to public health and is obligated to take more responsibility to ensure fair vaccine provision as a big power, they said.
RELATED ARTICLES:
Posted in: SOCIETY