Anthrax human immunoglobulin enters trials, important to China's defense against biological and chemical attacks
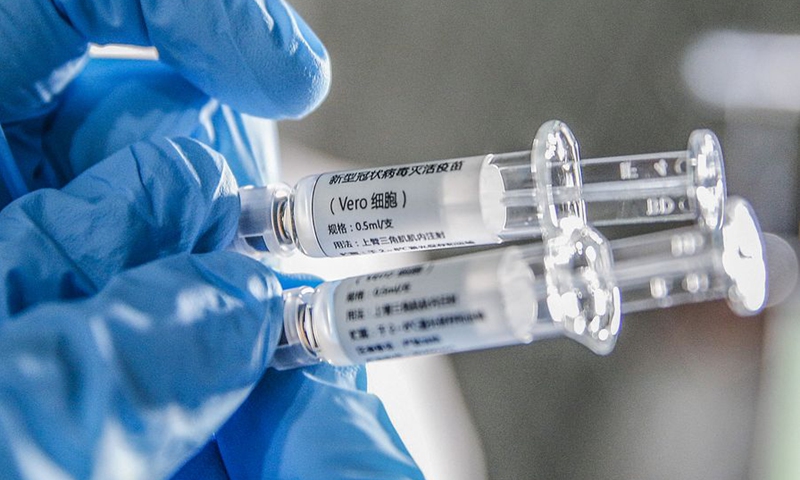
A staff member displays samples of the COVID-19 inactivated vaccine at Sinovac Biotech Ltd., in Beijing, capital of China, March 16, 2020. (Xinhua/Zhang Yuwei)
Top epidemiologist Chen Wei launched phase I clinical trials of the intravenous injection of anthrax human immunoglobulin in conjunction with a pharmacy company in Southwest China's Guizhou Province on Saturday.
Chen Wei, a researcher at the Institute of Military Medicine of the Chinese Academy of Military Sciences, said the trials hold significant meaning to China's defense against terrorism, biological and chemical attacks and public health emergencies, reported a Guizhou-based news agency on Saturday.
"From the perspective of national biosecurity, the prevention and treatment of anthrax is of vital importance," said Chen.
An immunologist, who asked to remain anonymous, told the Global Times on Saturday that anthrax is a severe infectious disease that is hard to control and is often used for biological and chemical weapons.
At present, the US is the only country in the world that has developed and produced anthrax human immunoglobulin products and has included the products into its national biodefense plan.
Previously, Chen, also a member of the National Committee of the Chinese People's Political Consultative Conference, had already called for promoting the development of national biosafety science in China during the two sessions in May.
Chen's team developed an adenovirus vector COVID-19 vaccine on March 16, the first domestically developed COVID-19 candidate vaccine to enter clinical trials.
Later on May 22, the vaccine also became the first in the world to disclose complete phase one clinical trial results and declare dual immune response in the recipient.