Russia approves world's first COVID-19 vaccine amid concerns over worsening epidemic in winter
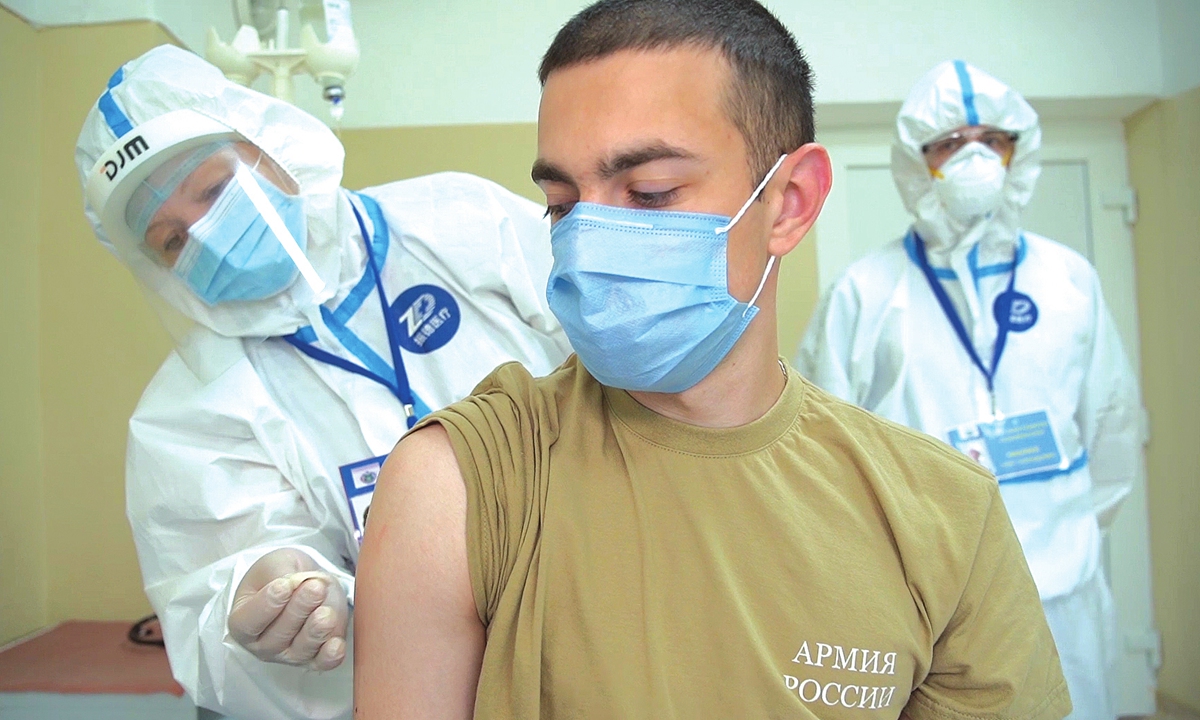
A volunteer takes part in a clinical trial of a COVID-19 vaccine in Moscow, Russia. Photo: VCG
Russia said on Tuesday it has approved the world's first COVID-19 vaccine and registered it with the national health department. Chinese experts believe the speed at which Russia is moving to roll out the vaccine reflects the Russia's concerns over the risk of a worsening epidemic in winter.
Called Sputnik V, the vaccine, developed by the Gamaleya National Research Center, has passed necessary tests and built immunity against the coronavirus, Russian President Vladimir Putin was quoted as saying at a Tuesday government meeting.
One of his daughters has already been injected with the vaccine, Putin said, noting that his daughter is feeling well and has a high level of antibodies.
The vaccine is expected to provide immunity from the coronavirus for up to two years, the Russian health ministry said.
Media reports said the vaccine is an adenovirus vector vaccine. It entered clinical trials in June, and completed phase 2 clinical trials on August 3.
No data on the trials has been released so far, triggering concerns over its safety and efficacy among some Western scientists. They questioned whether it is a little hasty to clear registration while phase 3 trials are still undergoing.
Russian officials told CNN in late July that they were compiling scientific data of the vaccine that would be made available for peer review and publication in early August.
The move is probably out of the Russian government's concerns over the risk of a worsening epidemic in winter, according to a Beijing-based immunologist who requested anonymity.
Russia has reported nearly 900,000 confirmed cases, with about 15,000 deaths as of Tuesday.
A recombinant novel coronavirus vaccine (Ad5-nCoV) candidate developed by a team led by Chinese military infectious disease expert Chen Wei and Chinese biopharmaceutical firm CanSino Biologics Inc. received a special one-year military drug approval in late June after phase 2 trials.
The anonymous experts said Russia is applying the similar mode to facilitate the application procedure of the vaccine, as scientists from various countries have warned of a second outbreak this winter.
As to the safety and efficacy of the candidate, the expert refused to comment, as no data had been released. But he noted that Russia has certain strengths in biological production research and development inherited from the Soviet era.
Tao Lina, a Shanghai-based vaccine researcher, estimated a 90 percent chance of success for the Russian candidate. He told the Global Times on Tuesday that it is unnecessary to be overly concerned as it must have passed proper tests.
Russian officials told Reuters in a previous interview that the country is eyeing massive vaccinations as early as October. The country plans to produce 30 million doses of an experimental COVID-19 vaccine domestically this year, with the potential to manufacture a further 170 million abroad.