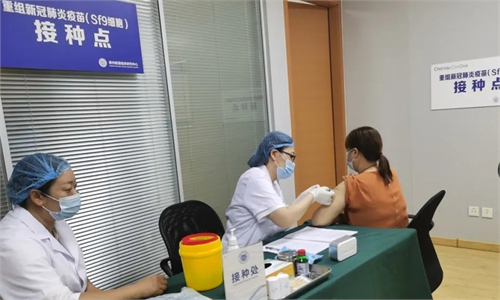
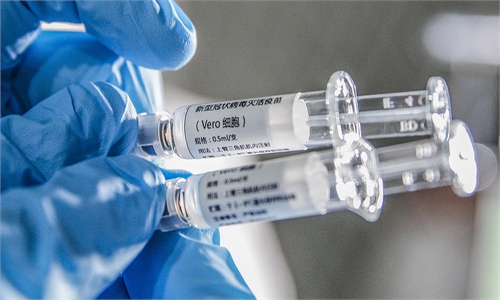
Chinese vaccines in sight, providing promising solution to conquer COVID-19
Only ‘one last mile’ before vaccines available to the public; no compromise on safety for speed
vaccine Photo: IC
A Chinese COVID-19 vaccine has been proven safe according to results from the latest phase III clinical trial in Brazil, reflecting the increased efforts China is making to advance vaccine development to make them available to the public as soon as possible.
Given that at least 60,000 people worldwide were vaccinated in the late-stage clinical trial, there is only one last mile before Chinese vaccines can be put on the market, which could be as soon as next week or next month, some experts forecast.
There were no serious side effects reported among the 9,000 volunteers in Brazil that were inoculated with a vaccine developed by Chinese vaccine manufacturer Sinovac, which shows the company’s candidate CoronaVac is safe, according to a statement Brazilian biomedical research center Butantan Institute sent to the Global Times on Tuesday.
The Brazilian government is set to include the Chinese vaccine in its national immunization program, having agreed to buy 46 million doses of the Sinovac vaccine, Reuters reported Wednesday.
Three vaccine manufacturers in China, Sinovac, Sinopharm and CanSino, have been operating clinical trials in dozens of countries such as Brazil, Turkey, Indonesia, UAE, Egypt, Russia and Pakistan, recruiting hundreds and thousands of volunteers.
With no severe adverse reactions reported, Chinese vaccines have proven safe, and Chinese authorities are working around the clock so manufacturers are fully prepared to roll out the vaccines for general public use, according to some Chinese officials.
Vaccines in sight
The final phase III clinical trial is the most crucial to prove a vaccine is safe and effective before it can be certified for public use, Wang Tao, chief reviewer at the Center for Drug Evaluation at the National Medical Products Administration (NMPA), told a press conference on Tuesday.
Besides late-stage trials to provide comprehensive data, authorities need to ensure that scale production of vaccines meets quality standards, Wang said.
An official from the NMPA who preferred not to be named told the Global Times on Wednesday that the authority has been working non-stop to ensure it can receive documents provided by enterprises anytime and provide feedback within 72 hours.
At the same time as streamlining approval processes, all necessary work ahead of the final vaccine licensing process has been prioritized by reviewing every procedure simultaneously to save time.
“Manufacturing conditions are mature, and the vaccines are ready. We’ll probably see them available to the public as soon as next month, or if not, by the end of this year,” a Beijing-based immunological expert who requested anonymity told the Global Times on Wednesday.
Shanghai-based immunology expert Tao Lina was even more bullish, suggesting that vaccines could be available as soon as next week, given that phase III clinical trial data will be soon released.
Once clinical trials provide sufficient research data in order to prove a vaccine is effective, stable and safe, and manufacturers can achieve scale production, companies can apply to put the vaccine onto the market, Wang from the NMPA said. And in accordance with the law and regulations, the NMPA will prioritize the COVID-19 vaccine application so it can be made available to the public as soon as possible.
As more Chinese officials and experts describe vaccine development in China as reaching the endgame, public enthusiasm has continued to grow in anticipation of the vaccines, considered the most promising solution to defeat the coronavirus which has caused over 41 million infections globally, with more than 1 million deaths.
Safety the top priority
While some Western media raised doubts over the safety of the Chinese vaccines as some claimed at the weekend that cities in East China’s Zhejiang Province are offering unproven vaccines to ordinary people by conducting a mass vaccination campaign, some experts see it as a misinterpretation of the emergency use of vaccines which is in line with national laws and has the support of the World Health Organization (WHO).
“Each country is sovereign and has the right to choose the tools and products that it considers to be best suited for its population. However, this choice must be guided by the highest standards of science and ethics,” Tarik Jaearevie, spokesperson of the WHO, told the Global Times on Wednesday.
The WHO has guidance around the exceptional authorization of vaccines and other products in the context of emergencies, Jaearevie noted.
Some Chinese officials also reiterated the importance of vaccine safety on Tuesday, which will not be compromised for the speed of development.
The emergency use strictly follows the review procedure in China as well as WHO rules, and has been reported to the WHO for ratification, Zheng Zhongwei, an official from the National Health Commission in charge of technology development, said at a press conference on Tuesday.
The WHO has issued target product profiles for COVID-19 vaccines outlining the minimum requirements of safety and efficacy, and also the requirements for the emergency use of vaccines.
As a member of COVAX, a global platform that supports the research and development (R&D) as well as manufacturing of COVID-19 vaccine candidates, China shares the principles and policies of the global organizations that are fighting the epidemic when it comes to securing access to safe and effective COVID-19 vaccines.
“Greater speed has been possible by making early investments in the scaling up and scaling out of manufacturing capacity, but not at the expense of safety assessments nor post-licensure processes, to assure strong ongoing safety monitoring,” a spokesperson for Gavi, which co-leads the COVAX Facility, told the Global Times via email.
Enhanced confidence
Responses from the Chinese public in a global poll on willingness to be vaccinated were the highest, meanwhile some foreign leaders have said they are willing both to be vaccinated with a Chinese vaccine and buy them for their country.
Philippine President Rodrigo Duterte recently said that he would “roll up his sleeves,” and his country would prioritize buying COVID-19 vaccines made by Russia or China, Bloomberg reported. Venezuela also reportedly said it plans to vaccinate citizens with Russian and Chinese coronavirus vaccines. Chinese vaccine manufacturers Sinopharm and Sinovac are now conducting phase III clinical trials in South America.
Respondents from China gave the highest proportion of positive responses when they were asked about whether they would take a “proven, safe and effective vaccine,” according to a global survey of potential acceptance of a COVID-19 vaccine, which was unveiled on Tuesday on nature.com.
While some Western media are reporting that China’s global push on vaccination is a form of “vaccine diplomacy,” Chinese officials said it is more appropriate to describe it as a highly needed global cooperation in the face of common challenges, as the country is fulfilling its commitment as a responsible major power.
The participation of China in the COVAX initiative will help increase the negotiation ability of enterprises, encourage them to lift production capacity and ensure the output of vaccine manufacturing, which means that vaccines will be affordable and available for developing countries, Zhao Lijian, spokesperson of the Chinese Foreign Ministry, told a press briefing on Wednesday.
“It shows China is adhering to the mindset of building a shared community in public health while continuing to push forward the vaccine as a global public good,” he said.