Photo:VCG
China is to give people in high-risk COVID-19 groups coronavirus vaccine first, aiming to build a herd immunity protection chain through an active immunization program, said a senior official of the national medical authority on Saturday.
The first to be inoculated include those working in cold storage food facilities, port quarantine, transportation, fresh food markets, medical workers, and those who are going to study or work overseas, Zeng Yixin, deputy director of the National Health Commission, said during a press conference on virus prevention held at the State Council Information Office on Saturday.
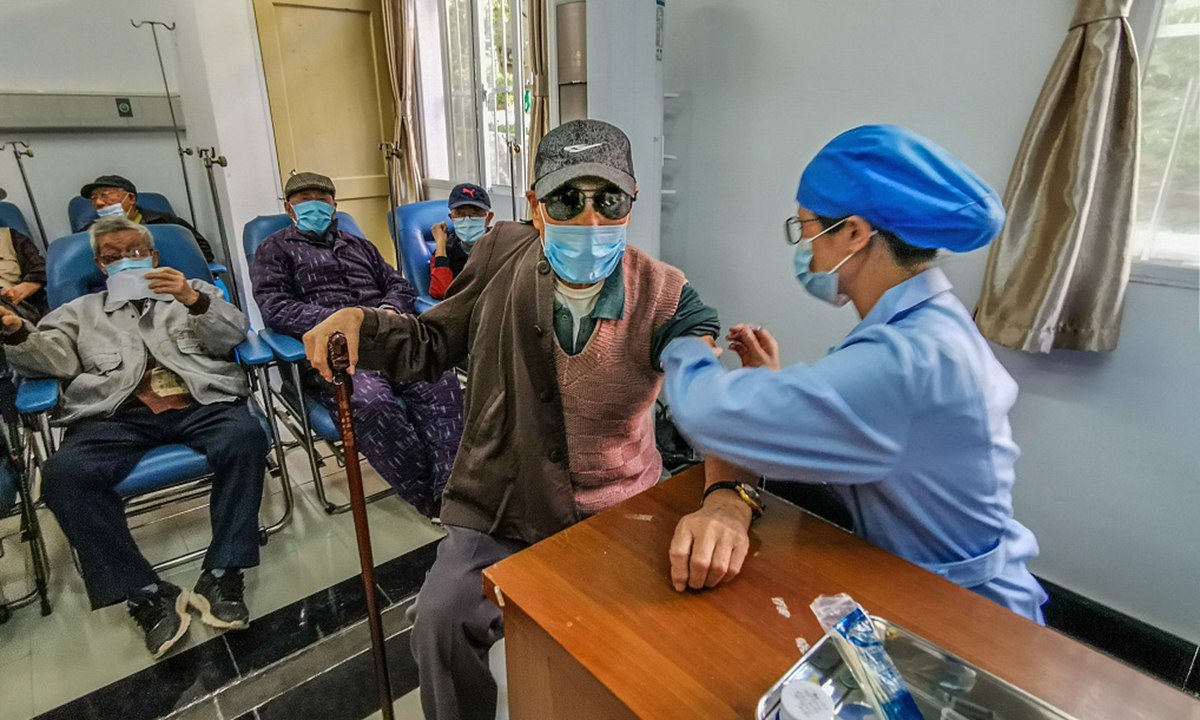
The elderly and those with existing health conditions will be next in line for a vaccination. The general population will be vaccinated as production capability gradually increases.
"Our goal is to establish a protection chain of herd immunity through active immunization," he noted.
China is planning to vaccinate 50 million people in high-risk groups before Chinese New Year which falls on February 12 to deter the spread of the virus during the upcoming travelling peak, the Chinese authority confirmed at an internal training session on Wednesday on potential mass inoculations.
Everyone in a high-risk will have their first injection of the vaccine before January 15, and their second by February 5.
All locations are required to complete training of health personnel who will deliver the vaccinations by December 20.
Three of China's leading vaccines, produced by Sinopharm and Sinovac, have been used in 13 provinces for emergency use.

The first shipment of 1.2 million doses of the COVID-19 inactivated vaccine called CoronaVac arrives in Indonesia on Sunday. Photo: Courtesy of Sinovac
China's five vaccine candidates are now undergoing phase three clinical trials in more than 16 countries.
If the progress goes well, China's inactivated vaccines are expected to be conditionally approved for market by the year-end or early next year, and with formal market approval as early as April, according to Wednesday's training session.
More than 1 million doses of COVID19 vaccines have been inoculated in China, and no severe adverse reactions have been reported. More than 60,000 people visited high-risk overseas regions after being vaccinated and no severe infection have been reported, according to the officials at the press conference.
A supervision mechanism by the national CDC has been established to monitor the adverse reactions to the vaccine in China.

vaccine Photo:VCG
Moreover, novel coronavirus mutations have been discovered in the UK, South Africa and other countries. Whether these mutations will affect the effectiveness of the vaccine has attracted much attention.
"The mutations of novel coronavirus are within the normal range and will not affect the effectiveness of the vaccine," Wang Huaqing, chief expert of immunization program of China CDC told the Global Times at the press conference.
Wang noted that virus mutations are a normal phenomenon, and generally do not affect the vaccine's ability to provide immunity to the virus.
"China also uses the antibodies produced by the vaccine developed in the early stage to conduct neutralization experiments on different strains of the virus, and the immune effect is relatively good, there is no change," Wang added.