Sinovac vaccine awaits Brazil emergency approval despite ‘unscientific’ comparison with mRNA

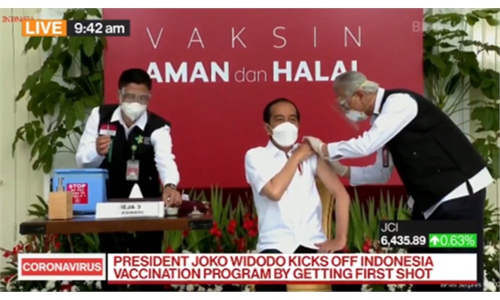
Indonesian President Joko Widodo (C) on Wednesday receives the country's first Covid-19 vaccine jab with the China-developed Sinovac shot at the Presidential Palace in Jakarta. Photo: VCG
Brazil's health regulator Anvisa on Thursday urged AstraZeneca and Sinovac-Butantan to submit further documentation required for their COVID-19 vaccines' getting emergency use approval on Sunday.
A source close to Sinovac told the Global Times on Friday that the company and its Brazilian partner Butantan have supplemented the documentation as requested.
Turkish President Tayyip Erdogan received the first dose of Sinovac's COVID-19 vaccine CoronaVac on Thursday, becoming the second country's president to use the CoronaVac after Indonesian President Joko Widodo.
Despite their vaccination reflects a trust in the shots and boosts higher confidence in CoronaVac, some Western media took aim at Chinese vaccines' efficacy while, as medical experts criticized, unscientifically putting CoronaVac in comparison with Pfizer and Moderna's mRNA vaccines, which reported over 90 percent protection rate.
Vaccine's efficacy varies so much in the design of clinical trials, in the selection of volunteers, and in the number of infected people, that comparisons cannot be made without discrimination, experts warned.
Shao Yiming from China's Centers for Disease Control and Prevention (CDC), who was involved in China's COVID-19 vaccine approval, told the Global Times on Friday that the Chinese drug authorities obtained the final-stage data as early as the end of October 2020, but maintained a very careful and detailed review procedure before conditional approval, partly due to data complexity.
According to the WHO Clinical Progression Scale, the scale provides a measure of illness severity across a range from class 0 (not infected) to class 10 (dead) with data elements that are rapidly obtainable from clinical records.
Sinovac's vaccine had a 50.4 percent efficacy if calculated with infected numbers in the trials at class 2 (symptomatic, but no assistance needed) and above.
But when researchers calculated the infected ones with class 3 (symptomatic, assistance needed) and above criteria, its efficacy can reach as high as 78 percent.
However, scientists generally believe that it is difficult to make a clear distinction between class 2 and class 3, and the description and definition of all symptoms depend mainly on volunteers' subjective judgment and description, which also brings great differences in conclusions.
Among almost all vaccines' trials globally, only two mRNA vaccines in the US have contrasted with the general clinical rule that the placebo group in the clinical study has a significantly higher incidence rate than the local natural rate, due to the researchers' close monitoring. This has led to suspicions that American researchers are applying a more rigorous standard in defining symptoms of volunteers in order to get better data on effectiveness, according to Shao.
However, researchers in CoronaVac's trials "chose the broadest number of symptoms, and like to capture even the simplest symptoms," Ricardo Palacios, the medical director of Butantan, said at the press meeting on Tuesday. "Those symptoms have not been included on similar studies. Our study was the one with the lowest class among all of the studies conducted."
Health care workers in the Brazilian trial were more sensitive to symptoms and more likely to amplify them than ordinary volunteers with other vaccines, according to analysts.
In addition, the environmental risks involved in different trials can also affect the effectiveness of the vaccine.
Palacios stressed on Tuesday that the trials in Brazil selected "the most challenging conditions for the vaccine," and he used a metaphor like "comparing runners on a flat surface versus a runner on an elevated terrain with obstacles." The challenges of this vaccine were the population involved - medical workers that are at risk of high exposure - which would decrease the vaccine's protection efficacy.
"We deliberated the choice of the most challenging conditions. Those tests would prove that the vaccine would work well under normal conditions," Palacios said.
Except for Coronavac, the other vaccines, including those developed by Pfizer, Moderna and AstraZeneca, were all tested on the general population, most of which require people aged 18 and older.
While Pfizer and Moderna's Phase III trials are almost mainly conducted and analyzed by the drug giants themselves, CoronaVac's trial in Brazil was conducted by the Butantan Institute and the results were analyzed and supervised by a third party, Shao noted. The whole process and results review are relatively independent from the producer.
"China is one of the first countries to receive Phase III clinical data in October. But unlike the US, vaccine manufacturers are not allowed to publish data without approval. China's drug administration aims to conduct a scientific and accountable assessment of the data in a condition free of public opinion pressure," Shao said.