China cautious to give seniors vaccine as provinces skip plan and await trials data for elderly
Provinces, cities skip previous plan to ensure safety of elderly

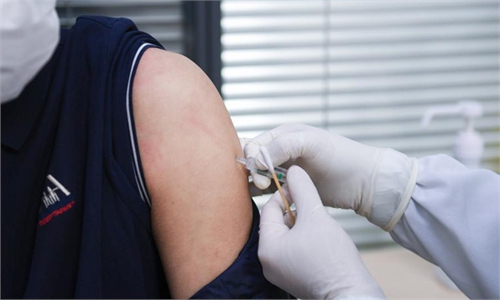
Medical workers check out people's health condition before vaccination at a temporary vaccination site in Chaoyang district, Beijing, on Monday. Photo: Li Hao/GT
As China planned to vaccinate 50 million people before Spring Festival, some Chinese cities started registering people from 18 to 59 but suspended the registration for elderly people, considering data of vaccine clinical trials for their age group has yet to be released, in sharp contrast to some Western countries that have been vaccinating seniors even though the Pfizer vaccine they used included very few seniors during clinical trials.
Chinese health experts believe that China is taking a cautious approach in vaccinating elderly people, especially after reports of seniors dying after receiving Pfizer's vaccine in Europe raised wide concerns - though there has been no evidence directly linking the deaths to the Pfizer vaccine.
China's vaccination is expected to cover senior citizens and people below the age of 18 by March as related data of the two age groups is likely to be released around mid-February, Chinese analysts said.
Residential communities in Beijing have started registering residents between 18 and 59 this week. An employee of a Chaoyang district community committee told the Global Times that the committee staff went door to door this week to write down the personal information of those who plan to receive the vaccine within the age range. They are expected to receive the vaccine after Spring Festival.
The Global Times learned that several provinces including East China's Zhejiang, North China's Shanxi and Southwest China's Sichuan provinces have also not included people aged 60 and above and those aged 18 and below in local vaccine registration process.
The Zhejiang provincial center for disease control and prevention told the Global Times on Wednesday that the two age groups were not eligible due to safety concerns as the data of the vaccine clinical trials on these age groups has not been released. But if people of the two age groups need to receive the vaccine for purposes such as going abroad, they could apply.
It seems that these places have skipped the previously issued vaccination sequence and put off registering elderly people. According to the National Health Commission, China's mass vaccination plan began with key groups in December 2020, and would then cover elderly people and groups with pre-existing conditions before being carried out among the general public.
Feng Duojia, president of the China Vaccine Industry Association, told the Global Times on Wednesday that elderly people are given priority over the general public in China, but since the related data of elderly people from clinical trials have not been released, it's understandable to start the vaccination campaign for the general public.
Yang Zhanqiu, a virologist at Wuhan University, told the Global Times on Wednesday that about half of seniors have pre-existing conditions who may develop severe side effects after vaccination, and China has to ensure their safety.
China is also learning lessons from Western countries' vaccination campaign, after the deaths in Norway and France, although there is no direct evidence linking the deaths to the vaccines, Yang said.
In France, elderly people were reluctant to receive the vaccine due to safety concerns. Radio France Internationale reported that 60 percent of French seniors in nursing homes did not accept the vaccination due to "skepticism over the safety of this new, rapidly rolled-out Pfizer vaccine."
Chinese health experts have called on Western countries to halt the use of the Pfizer vaccine for elderly people before the investigation on the deaths is completed.
China is especially cautious about elderly people's vaccination. Feng said that Chinese health authorities are probably working on detailed vaccination guidelines for elderly people, dividing them into different groups according to risks and age.
For example, people aged 70 and above with pre-existing conditions should be given priority over those aged 65, or relatively healthy elderly people, Feng said.
"Seniors will not have to wait too long for the vaccine. The clinical trial results on seniors are likely to be released around Spring Festival," Feng said.
Sinopharm's China National Biotech Group chairman Yang Xiaoming said in an interview with China Central Television that the company is submitting clinical trial data of children and teenagers to the National Medical Products Administration, and the vaccine campaign is likely to expand to cover people aged between 3 and 17 before March.
China has administered 22.77 million coronavirus vaccine doses as of Wednesday, and experts believe the country could finish vaccinating 50 million people from high-risk groups before Chinese New Year.
As for Western countries' choice of vaccinating the elderly people when studies of the vaccine have not included enough seniors, some Chinese health experts said that it could be because the spread of the coronavirus has not completely been controlled in Europe, and vaccinating elderly people could largely lower the death rate, Feng said.