China, Russia vaccine development offers hope to ease white-hot global vaccine scuffle
China, Russia joining hands to guarantee global vaccine supply: observers
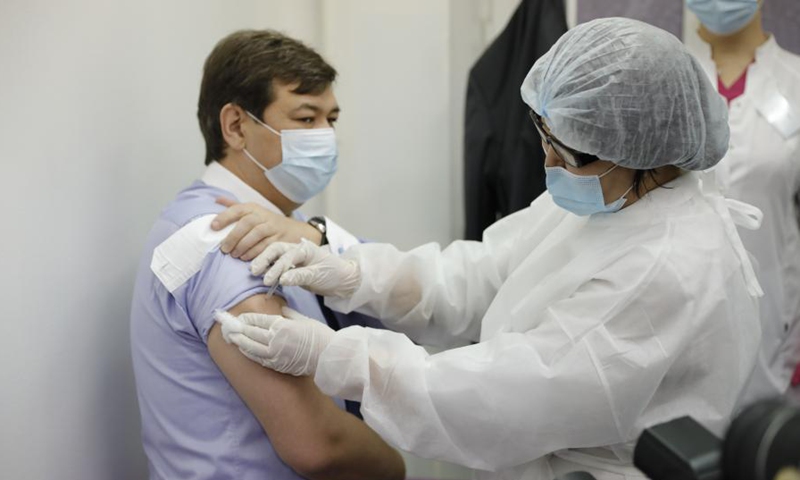
Kazakh deputy health minister Erlan Kiyasov receives Sputnik V vaccine at a local hospital in Nur-Sultan, Kazakhstan, on Feb. 1, 2021. Kazakhstan began its coronavirus vaccination campaign Monday using Russian-made Sputnik V. According to the ministry, the vaccination, carried out on a voluntary and free basis, will continue until the end of 2021 and will cover up to 6 million people. (Photo by Kalizhan Ospanov/Xinhua)
The impressive 92 percent protection rate of Russia's Sputnik V vaccine offers an opportunity for China and Russia, the two shepherds of global COVID-19 vaccine developers, to join hands on world vaccine distribution, help ease the white-hot global vaccine scuffle, and offer hope to control the pandemic, Chinese scientists said.
The study that followed a phase three trial of Sputnik V in Moscow hospitals and clinics that included nearly 22,000 participants was published in The Lancent international medical journal on Tuesday.
Sputnik V uses a snippet of DNA carried by a modified adenovirus, the same type of vaccine being developed by Johnson & Johnson and AstraZeneca, which show 66 and 70 percent efficacy rate, respectively.
There were 2,144 volunteers over 60 in the trial and the shots were shown to be 91.8% effective when tested on the older group, with no serious side effects reported that could be associated with Sputnik V, The Lancet summary said.
Chinese experts remain very positive toward Russia's vaccine. "The data is very promising, which means that Russia is one step closer to massively producing such vaccine," Yang Zhanqiu, a deputy director of the pathogen biology department at Wuhan University, told the Global Times.
But he also mentioned that the real effect cannot be entirely proven by a publication, and the real effect of the vaccine will only be proven after more vaccination results are revealed.
China has made substantial progress in its vaccine roll-out.
Chinese drug regulator approved the inactivated COVID-19 vaccine developed by Beijing Biological Products Institute under Sinopharm's subsidiary China National Biotec Group (CNBG) in late December. The vaccine showed 79.34 percent efficacy and a 99.52 percent antibody positive conversion rate. On Wednesday, the application of Chinese vaccine maker Sinovac for the conditional market launch of its inactivated vaccine was accepted by China's drug regulator.
On Monday, vaccine produced by Chinese biopharmaceutical company CanSino Biologics, who adopted the similar approach as Sputnik V, has met its pre-specified primary safety and efficacy criteria at interim analysis, with no serious adverse events, and the company will continue to advance its Phase-III clinical trials of the vaccine.
The technology to produce modified adenovirus is not quite mature, and Russia's production capacity may not allow it to massively produce the vaccine. This offers the opportunity for China and Russia to cooperate in the field to ease the white-hot global vaccine scuffle, a Beijing-based virologist told the Global Times.
In November, Shanghai-listed Tibet Rhodiola Pharmaceutical Holding Company said its subsidiary Topridge Pharma has entered into a deal on the registration, import, production and sales of Russia's Sputnik V coronavirus vaccine in China in a cooperation deal valued at $9 million.
It is highly possible China and Russia will join hands to massively produce modified adenovirus vaccines, because it has provided an effective alternative for inactivated vaccines and mRNA vaccines, and give the world more hope in controlling the pandemic, said the anonymous expert.
The global vaccine race has entered a white-hot stage where the EU has found itself stuck in speeding up the bloc's mass vaccination, due to the delayed delivery of AstraZeneca and Pfizer vaccines.
Meanwhile, more EU leaders have shown confidence in Chinese and Russian vaccines.
When asked about Russia's Sputnik V vaccine on Tuesday, French President Emmanuel Macron said that a few weeks ago he had sent a scientific mission to Russia and the exchanges were positive, and that there had been reports indicating the shots were effective against COVID-19. Germany's Federal Health Minister Jens Spahn also hinted on Sunday that vaccines from China and Russia could be used in Europe to overcome the current deficit of doses.
Previously, there were mounting Western media reports questioning the Russia vaccine's efficacy and data transparency, when it approved the vaccine for emergency use in August.
Now that the criteria for evaluating vaccines do not differ too much, Chinese and Russian vaccines are finding it hard to enter the European market because of political and scientific prejudice, said Yang.
Countries should keep an open mind on others' vaccine development and prioritize saving lives. "Any prejudice against a vaccine may cost lives in a pandemic," he noted.
China is ready and willing to offer vaccines to countries in need, and it depends largely on whether EU countries want to import Chinese vaccines, Feng Duojia, president of the China Vaccine Industry Association, told the Global Times.
Under the request of WHO, China will provide 10 million doses of COVID-19 vaccines for emergency use in developing countries, said Wang Wenbin, spokesperson for China's Ministry of Foreign Affairs on Wednesday.
So far, 15 other countries, including Argentina, Hungary and Serbia, have approved the Sputnik V vaccine for emergency use, media reported.