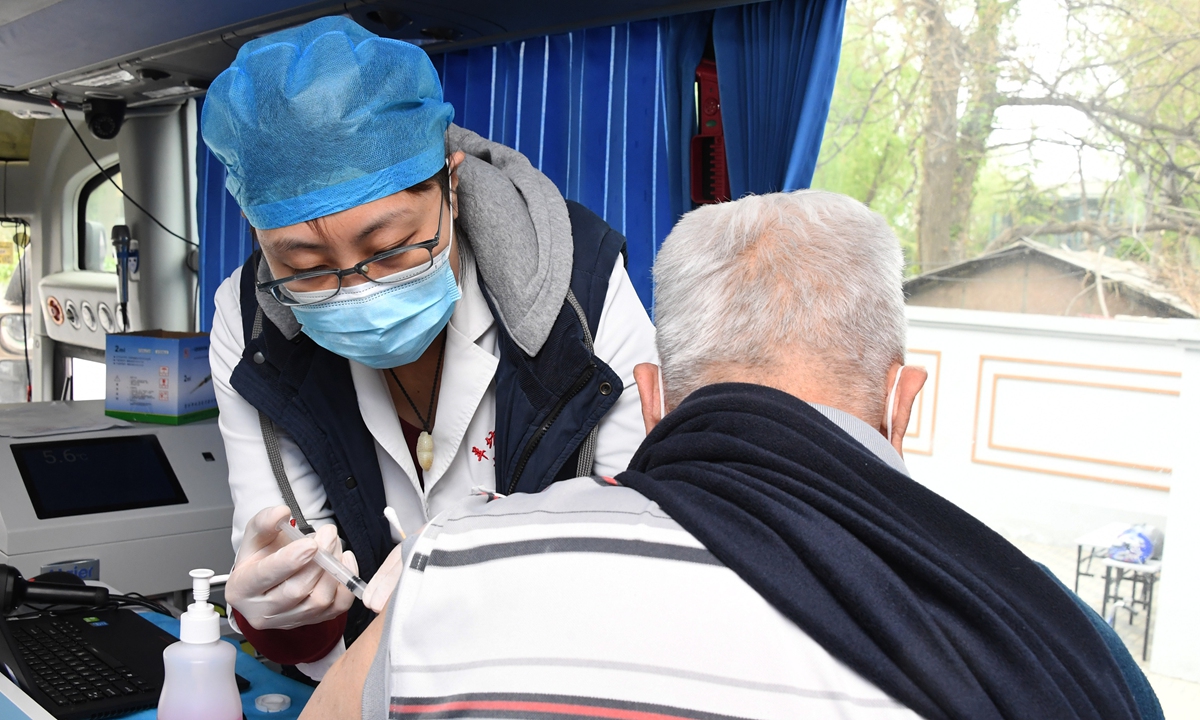
A resident of Beijing's Haidian district receives a dose of a COVID-19 vaccine at a mobile vaccination center that was created from a renovated bus. The capital city has administered more than 18 million doses as of Friday. Photo: cnsphoto
As the World Health Organization (WHO) is scheduled to announce the results of its evaluation of vaccines from Chinese producers Sinopharm and Sinovac Biotech for emergency authorization this week, the latest document uploaded by the WHO on the assessment of Sinopharm's vaccine showed that WHO experts have "overall confidence" in its ability to prevent COVID-19 while having "low confidence" on the risk of side effects for older patients.The document was interpreted by some overseas media, such as Reuters, as a new "proof" to question the vaccine's efficacy. In a report published on Wednesday, Reuters said in the headline that "WHO experts voice 'very low confidence' in some Sinopharm COVID-19 vaccine data" and explained that the data is in regard to the risk of serious side effects in some patients.
Chinese vaccine experts pointed out that the Reuters report amplified part of the WHO document's results, as the "some patients" it refers to are "older patients (beyond 60 years old)." The reason why the confidence is low is because the number of elderly people taking part in the trials was very limited.
They stressed that Sinopharm's vaccine has still been proven effective in the group aged 18-59, which means it is very likely to gain the WHO's approval.
The Sinopharm vaccine was authorized by 45 countries and jurisdictions for use in adults, with 65 million doses administered, according to media reports. The WHO document includes summaries of data from clinical trials in China, Bahrain, Egypt, Jordan and the United Arab Emirates.
Vaccine efficacy in multi-country phase III clinical trials was 78.1 percent after two doses, the document said. The efficacy in preventing hospitalization was 78.7 percent but the chance of preventing severity was "not estimated". Most adverse reactions were mild to moderate, with injection site pain, headache and fatigue being the most common.
The document also shows an analysis of safety among participants with comorbidities (was) limited by the low number of participants with comorbidities (other than obesity) in the phase III trial.
"Low confidence in some data does not necessarily mean the vaccine's efficacy is low or questionable, as the quality of the data would be affected by various elements such as the number of volunteers," Zhuang Shilihe, a Guangzhou-based vaccine expert, told the Global Times on Thursday.
The problem of clinical trials involving the Sinopharm Beijing institute vaccine is that few elderly people took part in the trials. But the WHO reports showed that the efficacy of the vaccine for people aged 18-59 is unquestioned and the safety of it is satisfactory, Zhuang said.
According to the WHO document, only 209 people beyond 60 years old participated in the phase III trial while participants aged from 18 to 59 were 13,556.
As there is a lack of clinical trial data to prove that Sinopharm's vaccine is safe and effective in older patients, he predicted that the WHO would grant approval with a limitation on being used on people aged 18-59.
The Sinopharm vaccine was effective among older people in Morocco, the Moroccan Health Ministry said in a statement in February. So far, only eight people in this age group reported minor adverse effects after receiving Sinopharm doses, which was equivalent to 2.2 cases per 10,000, it said.
Available data from the Moroccan vaccination campaign showed no increase in adverse effects among vaccinated people aged 60 and above, compared with others receiving the Sinopharm vaccine, the ministry said.
On Thursday, with the completion of the last tank of concrete, the COVID-19 Vaccine Workshop Phase III Project of the Sinopharm Beijing institute was successfully capped after 70 days of construction.
Once the project is put into use, the annual capacity will reach 3 billion doses.