Production line of mRNA vaccine co-developed by BioNTech and Fosun expected to be completed by August: Fosun chairman
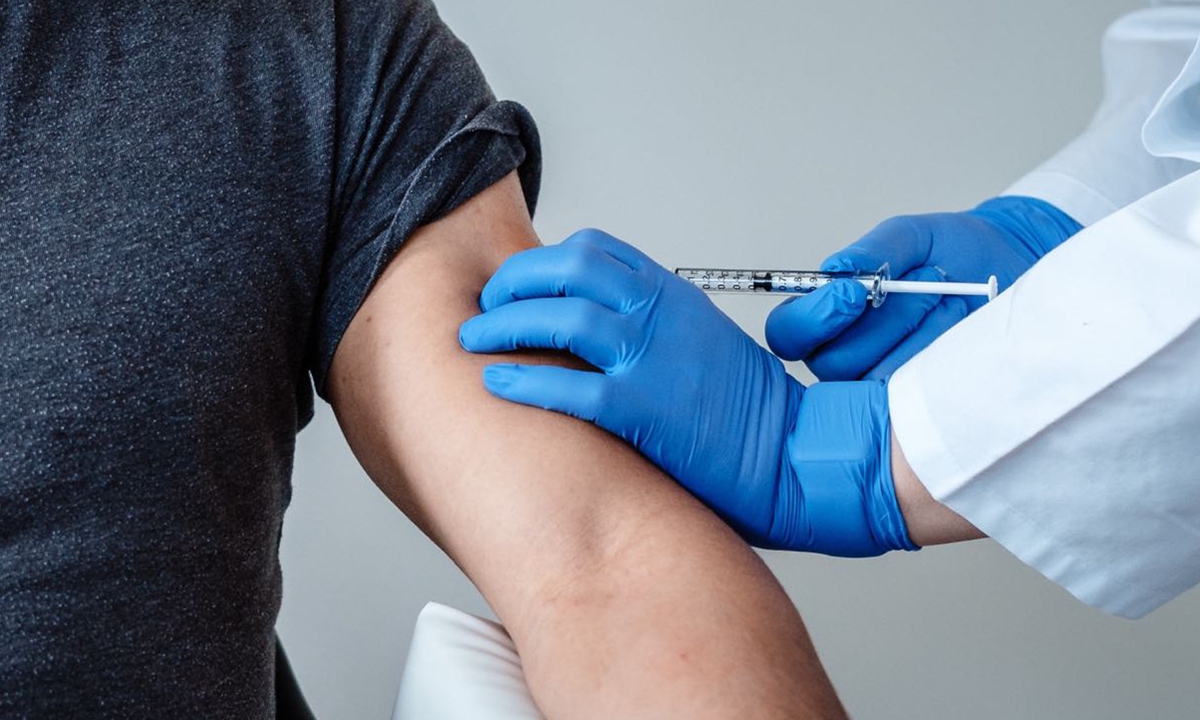
A dose of COVID19 vaccine BNT162b2 is given to a man. Photo: Courtesy of BioNTech
Production line of the COVID-19 mRNA vaccine co-developed by German-based BioNTech and China's Fosun Pharma is expected to be completed in China as early as August, which paves the way for mass production of the vaccine, said Fosun chairman.
The information was disclosed by Wu Yifang, the chairman at Fosun's shareholders' meeting on Friday. Wu said her company is working on technology transfer, purchase of supply chain equipment and preparation of raw materials with BioNTech. The first expert dispatched by German already arrived in Shanghai to remodel the factory, according to Wu.
The whole production line is expected to be completed and begin production in August, said the chairman.
Wu also said conditional pharmaceutical related approvals are nearly finished, and the company already has a clear picture of requests about follow-up research and clinic experiments.
The Global Times learned previously that Fosun Pharm has already submitted the clinical trial data and relevant materials to China's state regulator for reviews.
Experts said that approval of such imports could deepen the domestic vaccine pool and help facilitate reciprocal vaccine recognition.
Shao Yiming, China's leading physician-scientist and immunologist serving at the Chinese Center for Disease Control and Prevention, said that the imported mRNA vaccine will pave the way for mixed jabs in sequential inoculation, considering its efficacy.
The Fosun Pharma/BioNTech vaccine has been authorized for use or emergency use in multiple countries and regions including the US, the UK, Bahrain, Canada, Saudi Arabia and Mexico.
Global Times