Sinopharm subsidiary ready to deliver new COVID-19 drug based on recovered patients' plasma into clinical trials, vital for critical patients' treatment
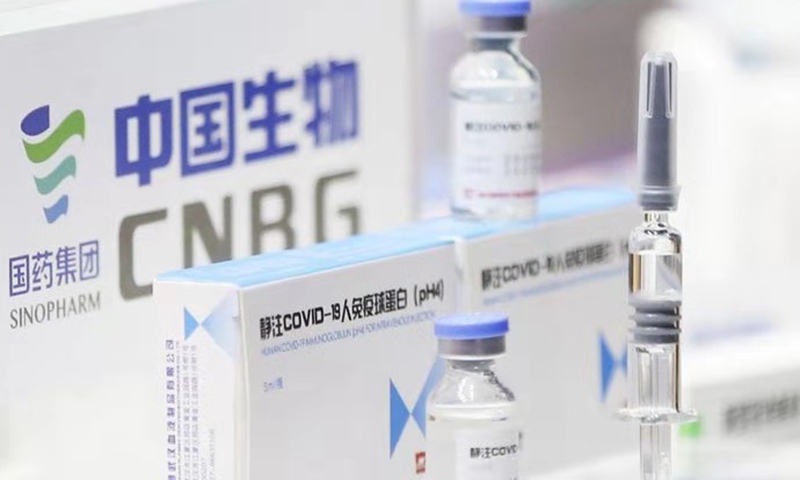
Sinopharm Photo:VCG
Chinese vaccine producer China National Biotec Group (CNBG), a Sinopharm subsidiary, announced that it has obtained approval of clinical trials for a COVID-19 drug based on human immuneglobulin developed from plasma of recovered COVID-19 patients, which experts said will be effective to treat critical patients.
The drug was unveiled at the 2021 China International Fair for Trade in Services (CIFTIS). CNBG said in a Friday announcement that it obtained approval of clinical trials on August 30.
In addition to the new drug, the CNBG also displayed other six anti-epidemic products, including two updated vaccines effective to combat mutations and another COVID-19 drug based on a monoclonal antibody.
The new drug contains high level of neutralizing antibodies to the novel coronavirus, according to the CNBG.
CNBG has been producing plasma products made of donated plasma from recovered patients since early 2020 and they were believed to be the most effective treatment on COVID-19 patients in severe conditions.
Chinese experts also took these products with them to treat local patients when they went to support foreign countries such as Italy.
The action principle of the newly unveiled drug is similar to previous plasma products. It uses antibodies to neutralize the virus, but would be more effective than the previous products, a Beijing-based immunologist told the Global Times on Saturday.
But it is uncertain how long the clinical trials will take as there are not so many patients in China now, the expert said on condition of anonymity.
The CNBG has not revealed where and when they would launch the clinical trials on the drug.
The expert noted that the drug should be limited to critical patients, as it may cost a lot, but would still be cheaper than monoclonal antibody drugs. He also warned that the drug may contain risks of infection of other viruses, such as HBV and HIV as well as other blood-borne communicated diseases.
The CNBG previously said that it has followed strict screenings to process the donated plasma.
Apart from routine tests, nearly 30 additional items will also be tested, including some common respiratory and intestinal bacteria as well as a pathogen to five kinds of infectious diseases like HIV, HBV, and syphilis. Virus inactivation treatment will also be conducted to improve safety of the plasma products.
According to the CNBG, it took at least a week from collecting plasma to preparing the products that can be used on COVID-19 patients. On average, one recovered patient can donate 400 milliliters of plasma to save two or three patients in critical conditions.