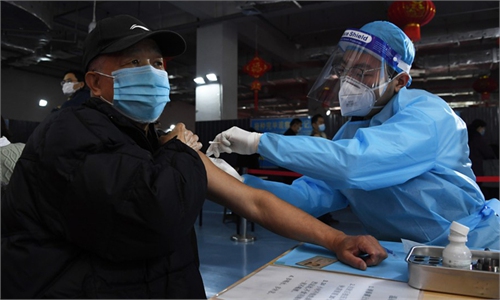
CanSino booster shot increases antibodies 78-fold
ReCOV becomes first Chinese vaccine conducting clinical trials in developed nation
CanSino's one-shot COVID-19 vaccine
Lab studies of China's booster shot continue to reveal promising immune efficacy, as the latest data show that taking a booster shot with CanSino's single-dose COVID-19 vaccine demonstrates an increase of at least 78-fold in average neutralizing antibody levels, with satisfactory safety condition.
Taking CanSino's recombinant adenovirus vector COVID-19 vaccine as a booster shot after two doses of inactivated jabs is proven to be safe and significantly more immunogenic than taking an inactivated vaccine as a booster, Zhu Fengcai, China's top vaccine clinical trials researcher, told the Global Times in a recent exclusive interview.
Zhu's latest co-written pre-print paper published on medRvix on Monday revealed more robust humoral and immune responses following the heterologous booster vaccination of CanSino's dose, compared with those following the homogeneous booster vaccination of Sinovac's CoronaVac. The peak of neutralizing antibody levels was achieved at day 14 after the heterologous boost.
No thromboses, vaccine-related anaphylaxis, or serious adverse events were seen in any cohort of participants, the paper said.
"A heterologous prime-boost regimen can increase the breadth, intensity and duration of the immune response, more than a homogeneous boost shot," Zhu told the Global Times.
Zhu's experimental Recombinant Two-Component Coronavirus Vaccine (ReCOV) against the coronavirus is undergoing phase I clinical trials in New Zealand and it's expected to enter phase II and III trials in about six weeks, said Zhu, also the deputy director of the Jiangsu Provincial Center for Disease Control and Prevention. He noted that this was the first time for full clinical trials of a Chinese-developed vaccine to be initiated in a developed country.
Zhu, working with top Chinese infectious disease expert Chen Wei, unveiled the world's first human clinical trials of a COVID-19 vaccine on March 16, 2020 in Wuhan, Central China's Hubei Province. Zhu told the Global Times that most of the earliest batch of volunteer recipients of CanSino's recombinant adenovirus vector vaccine have been given the booster shot.
Chinese health authorities clarified plans in early September to give a booster shot to high-risk groups including customs staff and individuals over 60 years old.
Although the exact plan for booster shots is still under research, Zhang Yuntao, vice president and chief scientist of Sinopharm's subsidiary China National Biotec Group (CNBG), previously told the Global Times that using different types of vaccines should be encouraged for booster shots. But specific vaccination schemes still require regulatory approval.
According to China's current COVID-19 vaccination guidelines, completing vaccination with the same type of vaccine product remains the mainstream protocol. More lab studies have proven the good performance of booster shots.
Chen Kun, a CNBG official, noted that experiments on animals showed that a booster six months after the second shot could increase the level of antibodies in recipients by five to 10 times, enhancing immunity effectiveness.
Another frontline producer Sinovac also revealed on Sunday that taking its inactivated vaccine as a booster can increase the potency, scale, and duration of anamnestic responses against SARS-CoV-2.
Zhu stressed that the prevalence of mutated strains around the world has brought great challenges to the first-generation vaccine, which was developed against the original strains. He said it was important to develop a second-generation vaccine against mutated strains.
A next-generation vaccine candidate against COVID-19 developed by Zhu is now undergoing phase I trials in New Zealand and is expected to enter the phase II and III trials in about six weeks.
As an improvement from the first-generation vaccine, the candidate ReCov can encode for the portions of the SARS-CoV-2 spike protein critical for neutralization, specifically the Receptor Binding Domain (RBD) and N-terminal Domain (NTD), and achieve a better level of immune response. RBD and NTD are the key subunits of the spike protein capable of binding to host cell receptors, or human bodies.
The technology was used in an mRNA vaccine developed by Moderna that has undergone clinical trials as well.
Zhu suggested that the efficacy of ReCov against the virus mutations requires further evidence in late-stage trials.
This is the first time that a Chinese-developed vaccine candidate has its clinical trials in a developed country, showing that Chinese vaccines have gained international recognition at the early trial stage, Zhu said.
"Until now, all of the Chinese [coronavirus] vaccines have been exported to developing countries. We hope this vaccine, if successful, will be approved for export in developed countries," Zhu was quoted as saying in a news report of the South China Morning Post.