CDC chief expert suggests vaccination for children under 12 after China hits 70% threshold
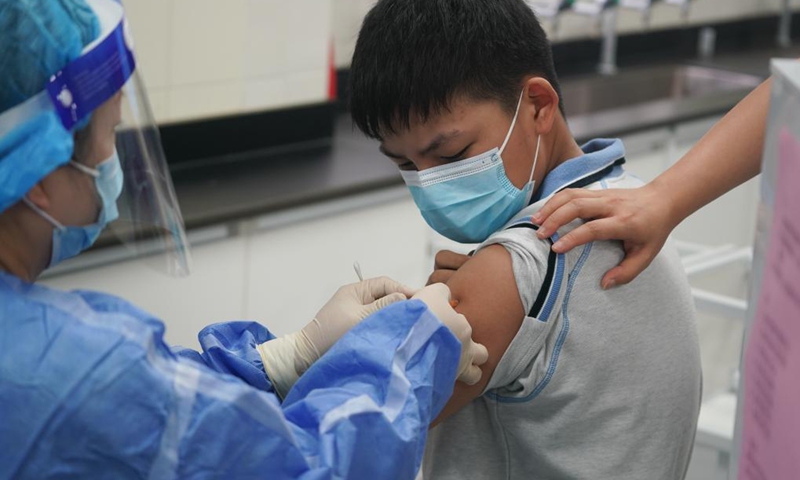
A student receives a dose of COVID-19 vaccine at a vaccination point of Wenshu middle school in Nanjing, east China's Jiangsu Province, Aug. 23, 2021. Nanjing started administering the first dose of COVID-19 vaccines among minors aged between 12 and 17. (Xinhua/Ji Chunpeng)
While Chinese parents of elementary school students worry about the safety of their children given the latest COVID-19 outbreak in schools of East China's Fujian Province, the chief immunologist from China's CDC on Thursday suggested health authorities expand vaccination to children under 12 years old, while also noting that the country had vaccinated more than 1 billion people aged 12 and above.
China now leads the world in administrating the highest number of COVID-19 vaccines, Lei Zhenglong, an official from the National Health Commission, said at a press conference on Thursday, noting that China is also one of a number of countries with very high vaccination rates.
China has a population of about 1.4 billion, according to the seventh national population census.
To vaccinate the more than 1 billion people, China had administered nearly 2.2 billion doses of COVID-19 vaccines, Mi Feng, an NHC spokesperson, said at the Thursday press conference.
Wang Huaqing, chief immunologist of China's CDC, said at the same press conference that the vaccination of more than 1 billion people lays a good foundation for China to control the epidemic. He noted that previous experience demonstrated the vaccines can exert better protection effectiveness with higher vaccination rate.
Although the highly contagious Delta variant is challenging the work of building herd immunity, the authorities would enhance virus and disease monitoring as well as continue to improve immunity strategy and vaccine development, Wang said.
The Delta variant has sparked three localized outbreaks in China this year, including the latest in Fujian Province.
So far China has approved three inactivated vaccines for emergency use on people aged 3 and above. However, based on a gradual vaccination strategy, shots now are only provided to people aged 12 and above.
According to Lei, among the fully vaccinated people in China, more than 200 million are aged 60 and above and another 95 million are aged 12-17.
Wang called for more people at the vaccine-accessible age range to get vaccinated as soon as possible. He also suggested expansion of vaccination to children under 12.
Over 40 percent of confirmed cases in the latest COVID-19 resurgence in Putian, Fujian, are minors under 12, local authorities revealed Thursday.
Over half of the locally confirmed cases in this round of the epidemic were teachers and students in kindergartens, elementary and middle schools, and factory employees. Cluster infections are obvious in the latest outbreak, according to Mi.
The infections in schools raised concerns among parents over the safety of the children.
The Fujian epidemic, in the eyes of many, represents appropriate timing for China to expand vaccination to children aged 3-12, Tao Lina, a Shanghai-based expert, told the Global Times on Thursday.
In response to concerns over the safety of the vaccines for minors, Tao said that inactivated vaccines are very safe for children and have already been widely used among young population groups.
After the authorization for use on adults, inactivated vaccines produced by China's Sinopharm and Sinovac were tested as also being safe for the 3 to 17 age group after clinical trials and expert reviews, and were authorized for emergency use by that age group by the relevant authorities, officials revealed in June.
According to a study published on The Lancet on Wednesday, the inactivated COVID-19 vaccine developed by Sinopharm's institute in Beijing is safe and low-risk at all tested dose levels in participants aged 3-17 years during the Phase I/II clinical trials. The vaccine also elicited robust humoral responses against SARS-CoV-2 infection after two doses.
According to Phase II of the clinical trials, Sinovac's COVID-19 vaccine is at least 98.9 percent effective in producing antibodies in that age group, a higher efficacy than in population over 18 years old.