Chinese oral anti-COVID drug delivered to epidemic-hit regions, ‘effective’ in inhibiting virus
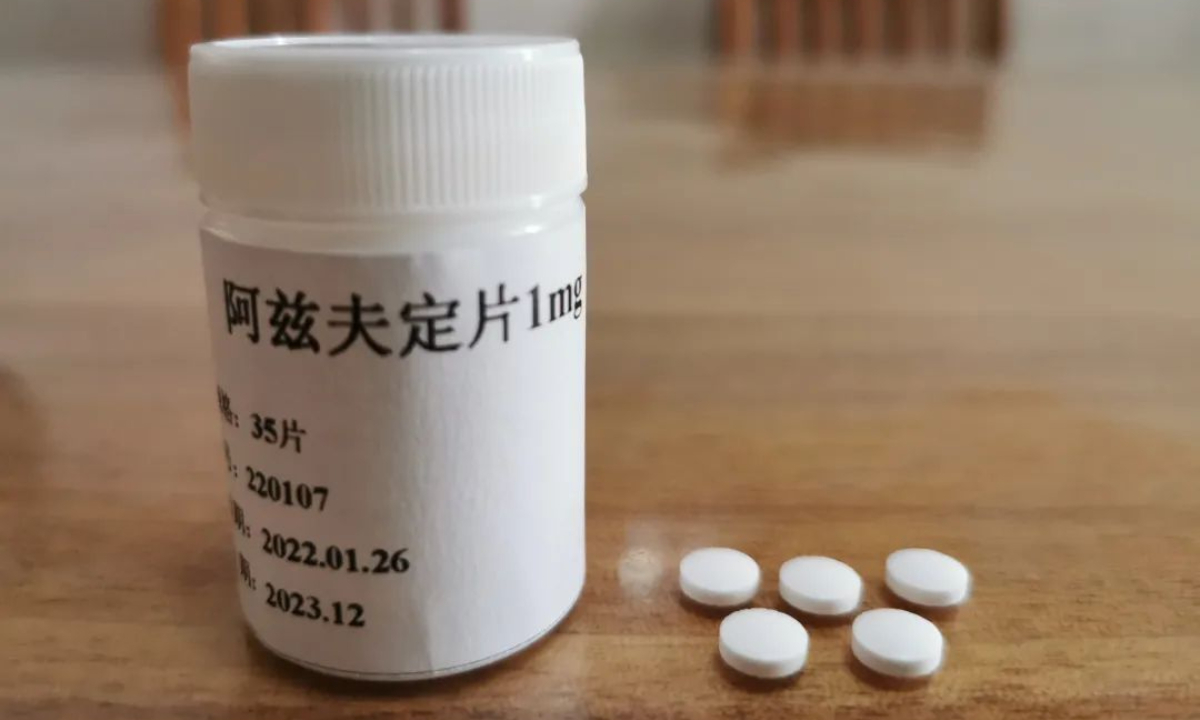
China-developed oral drug for COVID-19 treatment Azvudine
China-developed oral drug for COVID-19 treatment Azvudine has been delivered to various epidemic-hit regions, including Central China's Henan, South China's Hainan, and Northwest China's Xinjiang region, according to the company.
Experts noted on Friday that small-molecule antiviral drugs are effective in curbing the virus and should be widely promoted in the long run, even though the country continues to adhere to the dynamic zero-COVID strategy and use prompt prevention and control measures to curb the virus' spread as fast as possible.
The country on Thursday registered 648 local cases, including 595 in Hainan, and 1,203 asymptomatic carriers, including 614 in Hainan, 410 in Xinjiang and 20 in Southwest China's Xizang, according to the National Health Commission.
In Hainan alone, as of 12 pm on Friday, this wave of flare-ups starting from August 1 has seen 5,154 cases and the epidemic in the province is developing rapidly, Hainan authorities said at Friday's press briefing.
Hainan has built makeshift hospitals with nearly 9,000 beds so far, and more than 11,000 medical staffers from 19 provinces and cities have arrived to support the fight against the virus, with a capacity of nucleic acid test samples of 1.97 million tubes per day, according to the authorities.
Fosun Pharma said in a statement it sent to the Global Times that Azvudine, produced by Genuine Biotech, based in Central China's Henan, has been delivered to some epidemic-hit regions. Every bottle of Azvudine is priced at 270 yuan ($40), with each containing 35 pills, according to the statement. It's reported that the drug could be covered by medical insurance temporarily.
Azvudine is the first small-molecule antiviral drug for COVID-19 treatment approved in China with completely independent intellectual property rights, and the drug can inhibit the activity of the coronavirus, said the statement.
China's drug regulator on Tuesday released a notice to include Azvudine into the ninth edition of diagnostic and treatment protocols for the novel coronavirus for common-type COVID-19 adult patients.
The National Medical Products Administration in February this year granted conditional approval for imports of Pfizer's Paxlovid COVID-19 pill, and the China-developed neutralizing antibody combination therapy BRII-196/BRII-198 was approved for the market in China in July.
Pfizer's Paxlovid COVID-19 pill is priced at 2,300 yuan per box, with each box having 30 pills, which can meet a five-day treatment plan. The drug has been covered by medical insurance.
Both the drugs are "effective" in curbing the virus, and their treatment targets are "different," Lu Hongzhou, head of the Third People's Hospital of Shenzhen, told the Global Times on Friday.
The latest flare-ups in Hainan, Xizang and other regions will be contained under effective preventive measures, including high-frequency nucleic acid tests, which could detect possible cases in a fast manner. For the long term, in addition to regular epidemic prevention and control, research will be focused on the development of effective nasal spray vaccines, as well as small-molecule antiviral drugs, Lu said.
Antiviral drugs have been used in some hospitals in Shenzhen, as they're effective in "inhibiting the virus at an early stage," which should be "widely promoted," Lu said. After curbing the virus, the infected patients will not be infectious and they will not move into serious or critical condition due to COVID-19, and the virus will not lead to further damage to organs, according to Lu.
A medical staffer from a Xinjiang hospital said most of the hospitals haven't started to use the drugs, as they have just arrived in the region.
Lu stressed that it would be better to use the drugs as early as possible.