1st China-developed ECMO approved for market use as a latest effort to strengthen treatment for COVID-19 patients with severe illness
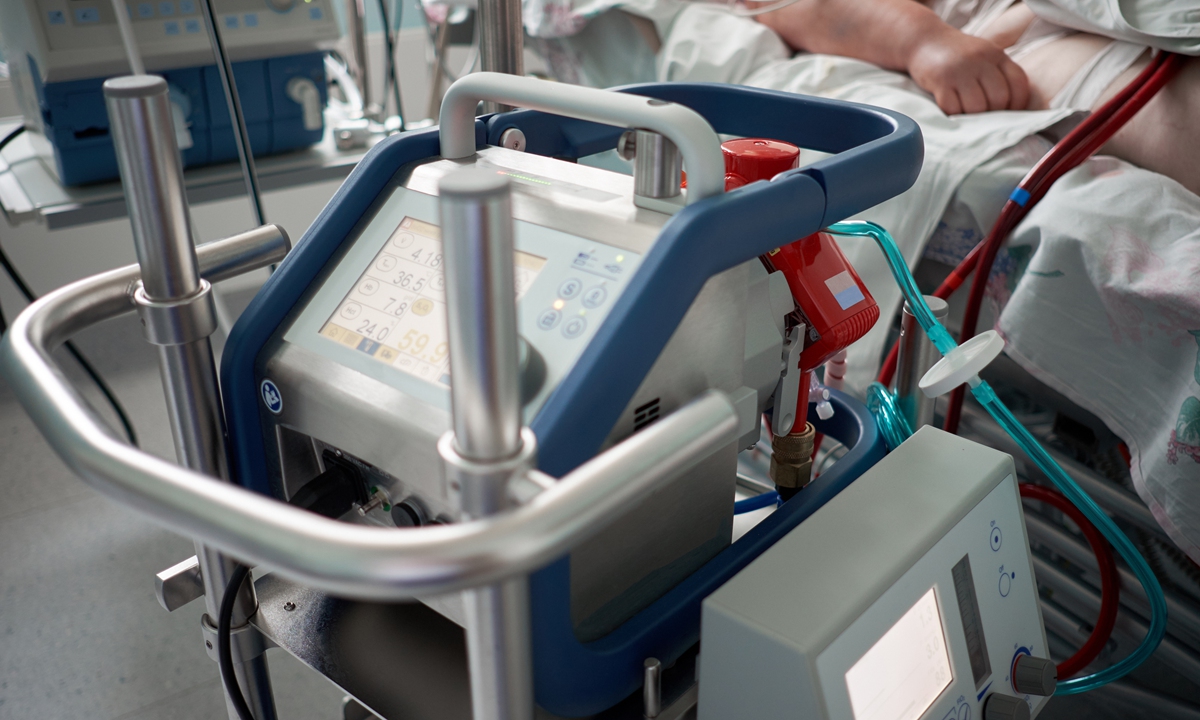
Working ECMO machine in intensive care unit Photo: IC
The first China-developed "artificial lung," the extracorporal life support product, was approved for emergency market use for adult patients with a predictable risk of continued deterioration or death, National Medical Products Administration revealed on Thursday, adding to the country's latest efforts to optimize medical resources and strengthen treatment capacity for COVID-19 patients with severe illness while the Chinese localities are currently experiencing the peak of Omicron infections.
In a bid to ensure the treatment of severe cases of COVID-19, China's National Medical Products Administration made the emergency approval for the extracorporeal membrane oxygenation (ECMO) product for adult patients with acute respiratory or acute cardiopulmonary failures and whose conditions are difficult to control with other treatments and are at a predictable risk of continued deterioration or death, the administration announced on its official website on Thursday.
As China's first domestic ECMO and accessories kit, it basically reaches the international level of similar products in its performance indicators, the administration said.
Lu Hongzhou, head of the Third People's Hospital of Shenzhen, who led the clinical test of the equipment before it hit the market, told the Global Times on Thursday that the equipment had a reliable performance during the test. The expert of critical care medicine also thinks highly of the domestically produced product for its high cost-performance ratio.
The ECMO product is salvage treatment equipment for critical patients who have not responded to conventional treatment. Domestic products will play an important role in meeting the urgent clinical needs and ensuring the treatment of severe COVID-19 patients, according to the administration, which vows to strengthen supervision of the product and safe use of it.
An increasing demand for emergency medical resources for critical cases has emerged across the nation since the beginning of January as Chinese localities are experiencing the peak of Omicron infections in addition to the large population base of the country.
Jiao Yahui, a senior official from the National Health Commission (NHC), said during a press briefing on December 27, 2022 that in terms of the rescue equipment for critical patients, China had 167,000 sets of hemodialysis equipment as of December 25, 24,000 pieces of continuous renal replacement therapy (CRRT) equipment, over 2,600 ECMOs, 131,000 invasive ventilators, 157,000 non-invasive ventilators, 1.09 million monitors, and 58,000 high-flow oxygen inhalers in China as of December 25, 2022.
ECMO can provide continuous extracorporeal respiration and circulation for patients with severe cardiopulmonary failure to win valuable time for the rescue of the patients. Since the start of the COVID-19 epidemic, ECMO has been gradually known to the public, whereas its core and key technologies have long been monopolized by overseas companies, the equipment and accessories are expensive.
The price of ECMO equipment ranges from 1 million yuan ($145,201) to 3.5 million yuan and the costs of using the equipment is as expensive as 200,000 yuan for two weeks, according to media reports.
Xinhua reported in April 2020 that domestic manufacturing of ECMO equipment faces key materials and technologies barriers.
Apart from the newly approved ECMO produced by Chinabridge Medical, more domestic products are undergoing clinical research. The ECMO equipment jointly developed by The First Affiliated Hospital of Xi'an Jiaotong University and the College of Biomedical Engineering of Sichuan University and the School of Mechanical Engineering of Xi'an Jiaotong University entered the clinical test phase since November 8, 2021, according to media reports.