Chinese scientists develop stretchable electrodes for invasive BCI, breaking key bottleneck
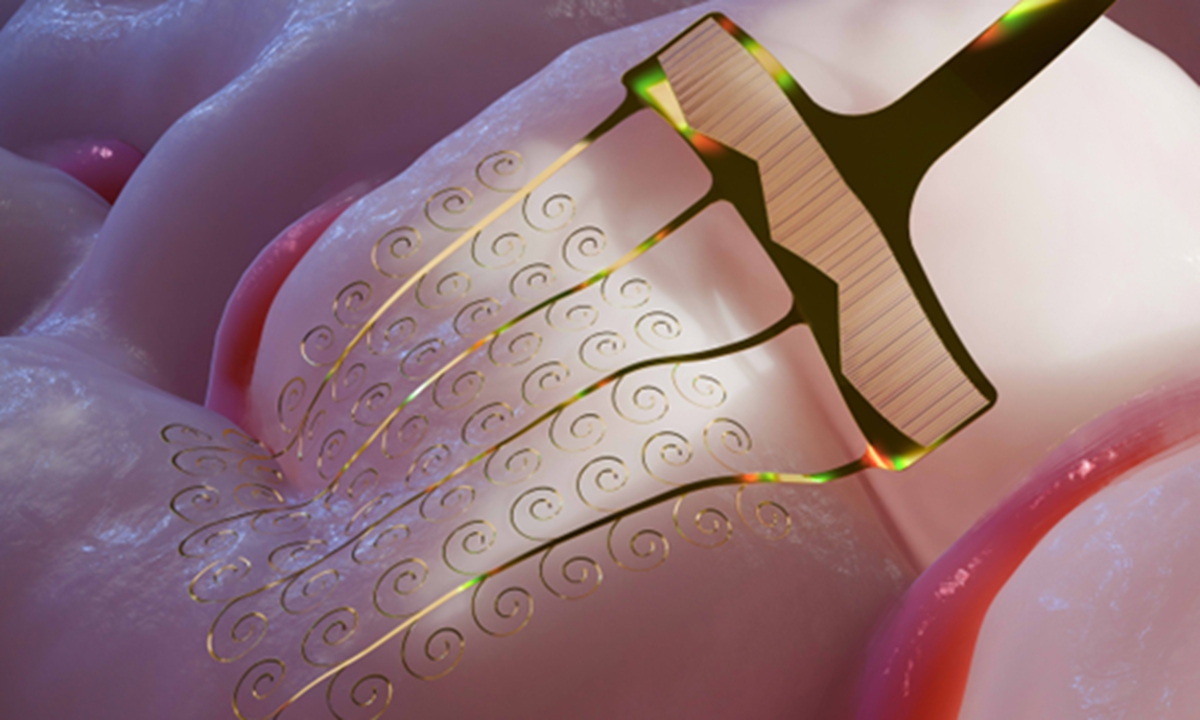
Illustration of a high-throughput stretchable flexible microelectrode. Photo: Courtesy of BCIFlex
A Chinese research team has developed a stretchable, flexible microelectrode that can dynamically adapt to brain motion, addressing a long-standing bottleneck in invasive brain-computer interface (BCI) technology in which conventional flexible electrodes are prone to displacement and retraction during brain movement.The findings, led by Fang Ying, a senior researcher at the Chinese Institute for Brain Research Beijing, were published on Thursday in Nature Electronics, a sister journal of Nature. The study reports a stretchable flexible electrode that combines high-throughput neural signal acquisition with biomechanical compliance.
Researchers said the new electrode architecture provides a foundational solution for the long-term stability of invasive BCIs by allowing implanted electrodes to mechanically conform to the brain's natural pulsation and intracranial displacement, significantly reducing the risks of signal loss and tissue damage.
Fang told the Global Times in an exclusive interview on Saturday that the research was motivated by problems observed several years earlier during invasive BCI experiments in non-human primates.
"About four years ago, we found that flexible electrodes carried a real risk of retraction due to brain movement," Fang said. "That prompted us to explore new approaches to reduce the risk of electrodes being pulled out when one end is anchored to the brain and the other is fixed to the skull."
The issue gained wider attention in 2024 after US entrepreneur Elon Musk's company Neuralink carried out its first invasive BCI clinical trial in a human patient. However, around 85 percent of the 1,024-channel flexible electrodes implanted in the patient retracted from the brain within weeks of implantation, largely because the electrodes were bendable but not stretchable and could not accommodate natural brain motion.
Facing the brain's constant pulsation and intracranial displacement, traditional linear electrodes struggle to adapt in real time, making them prone to shifting or even being pulled out of neural tissue entirely. Such detachment not only reduces the number and accuracy of neural signals collected but can also trigger inflammatory responses in brain tissue.
As a result, developing new flexible electrodes capable of adapting to brain dynamics and enabling long-term stable signal recording has become a key hurdle to the clinical application of invasive BCIs, Fang's team explained in an written response to the Global Times.
To address this industry-wide challenge, the team proposed a high-throughput, stretchable electrode architecture. According to the study , the design leverages the extremely low bending stiffness of ultrathin flexible films, redirecting tensile stress into low-energy buckling deformations. This allows the electrodes to dynamically follow brain pulsations and intracranial movements after implantation, ensuring long-term stability within neural tissue.
Fang explained that the core innovation lies in the electrode's spiral structural design.
"We designed the electrode as a coiled structure. Because it is ultrathin, it bends very easily," Fang said. "Through structural design, we convert stretching into bending and twisting of the electrode itself, which dramatically reduces the force required for stretching. After implantation, the electrode can move up and down with the brain's natural pulsation, preventing displacement or retraction like what we saw with Neuralink's electrodes."
The stretchable electrodes are also significantly softer than conventional linear designs, Fang emphasized. She noted that stretching Neuralink's linear electrodes by 100 micrometers requires a force of about 4 millinewtons, whereas the new stretchable electrodes require only 37 micronewtons—roughly one-hundredth of the force. This substantially lowers mechanical stress on brain tissue and, at the source, helps avoid immune responses and glial scarring commonly associated with traditional linear electrodes.
To verify implantation reliability and long-term stability, the team conducted systematic tests in monkeys. The results showed that the stretchable flexible electrodes enabled long-term, stable neural recordings in the primate brain.
To further assess large-scale signal acquisition capability, the researchers successfully implanted a 1,024-channel high-density stretchable electrode array in a primate brain—a scale comparable to Neuralink's core specifications. The system achieved large-scale, high-quality neuronal signal recordings, further validating the performance advantages of the stretchable design, according to the research document.
BCI technology, which establishes a direct information exchange channel between the brain and external devices, is widely seen as a pathway toward deeper integration between human intelligence and artificial intelligence. Major countries and regions are accelerating their research in the BCI sector, and China has included BCI development in recommendations for its upcoming 15th Five-Year Plan, underscoring strong national-level support for the field.



