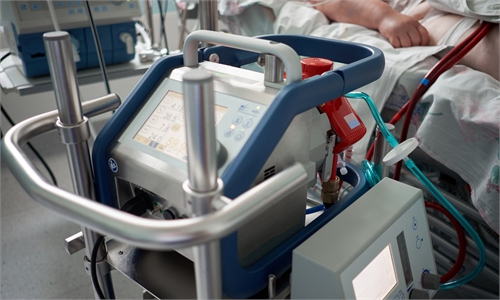
China's first homegrown ECMO with full system makes impressive progress, to enter market in 2024
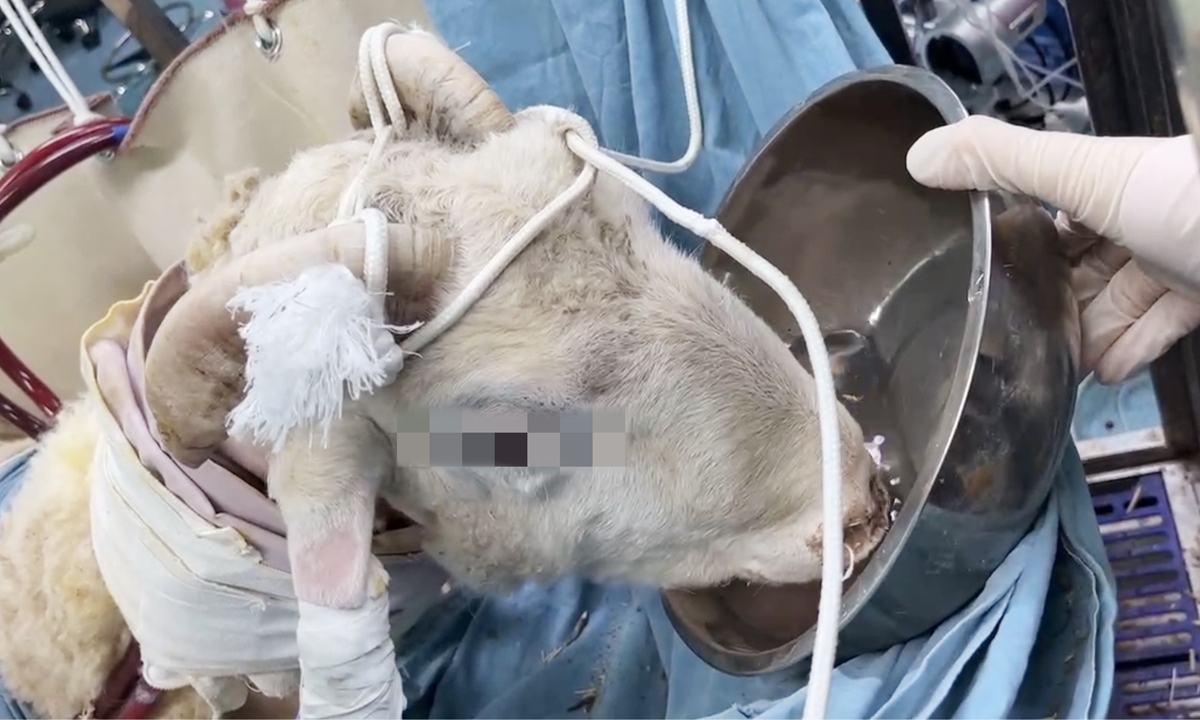
Photo:Courtesy of Beijing Aerospace Changfeng Co
Tony the sheep had been living with the support of a heart-lung bypass machine for 14 days as of Friday, marking a big success in the animal testing phase for China's first-ever whole-system ECMO development, insiders revealed to the Global Times on Sunday.
ECMO stands for extracorporeal membrane oxygenation, which pumps and oxygenates a patient's blood from outside the body, allowing the heart and lungs to rest. It is used when a patient has heart or lung failure and often during open-heart surgery, or liver and lung transplants. ECMO has gradually earned its reputation of being a "miracle machine" in China since the COVID-19 epidemic broke out in Wuhan, Central China's Hubei Province at the end of 2019. Stories of critically ill COVID-19 patients who survived on ECMO devices made front-page news.
However, the miracles do come with a big price, which is generally unaffordable. An imported ECMO machine is priced from 1 million yuan ($149,800) to 3.5 million yuan. It includes a complex circuit of pumps, tubes, filters and monitors. Generally, the cost of two weeks of treatment could be as much as 200,000 yuan, according to media reports.
The ECMO machine on trial is codenamed ACM9000, and has been jointly developed by more than 130 Chinese scientists and researchers in the past three years, said Su Zihua, CEO of Beijing Aerosapce Changfeng Co, who leads the China's National Key R&D Program (NKP) approved by the Ministry of Science and Technology in 2021.
Scientists and researchers from Beihang University and medical experts with top-notch hospitals such as Fuwai Hospital of the Chinese Academy of Medical Sciences and Beijing University Third Hospital have also participated in the project.
"The successful animal testing with a prolonged testing period fully demonstrates that we have overcome all the technological barriers [in developing ECMO] and we are now working to further optimize it," Su told the Global Times.
The ACM9000 ECMO is expected to attain the license for formal clinical application by 2024. Before that, the model will undergo more animal testing, clinical trials and ethical and data examination.
Clinical trials will be carried out in Fuwai Hospital of the Chinese Academy of Medical Sciences and Beijing University Third Hospital, which are facing the gravest challenge from COVID-19 treatment pressure, Su revealed.
When asked why a sheep was picked as the experiment animal, Wang Yawei, the deputy chief of the project, said that it is standard to use sheep when developing ECMO devices worldwide.
The sheep in ECMO animal tests usually weigh 50 to 70 kilograms, which is similar to an adult human being on average. More importantly, the sheep's blood system is more vulnerable than in humans, with hemolysis more likely to develop. That is to say, if a sheep can survive for a certain period with the support of ECMO, it would probably be safe for human patients, Wang said.
This is the first time that a full-chain ECMO system including all sensors has been wholly developed by China, and the development aims to help those patients who suffer from critical sickness and heart-lung faliure, Wang stressed. From the monitors, key sensors, to parts including pumps, tubes and filters, the ECMO is completely domestically developed.
Reading of the sheep's vitals including the amount of red blood cells has shown that the key component of the Chinese homegrown ECMO - the centrifugal pump — shows outstanding compatibility with blood.
There were also good results with the key component for air-blood exchange, the artificial membrane. No blood clots developed in the process and the resistance in the blood flow was low.
The ACM9000 has shown performance comparable to the most advanced foreign products, and in some key areas it is even better, Wang said.
Su said he expected that the cost could fall by at least 30 percent after the ACM9000 officially enters the market, greatly saving expenses for Chinese patients.
Due to the hefty price, the machine is only used at the very end of the medical treatment. The cheaper the ECMO becomes, the sooner it could actually be adopted, expanding the scope of the ECMO use and increasing the chance of survival for patients, Wang noted. "Our goal is to greatly reduce the patient's spending on ECMO compared to an imported product."
"It is complicated to develop ECMO as it involves dozens of parts. However, we have managed to achieve optimization at every part. And the result [of the animal testing] is better than what we expected," Chen Zengsheng, a scientist from Beihang University, told the Global Times. Chen has a background of research in the field of artificial organs oversees and joined the Chinese ECMO R&D project after coming back to China in 2019.
According to Chen, apart from the civilian use of ECMO, Chinese scientists are also mulling expanding the working scenario for ECMO to the battlefield. To meet military use requirements, the scientists are also working to ensure ECMO performance in low-temperature and plateau environments, and downsizing the machine is one of their goals.